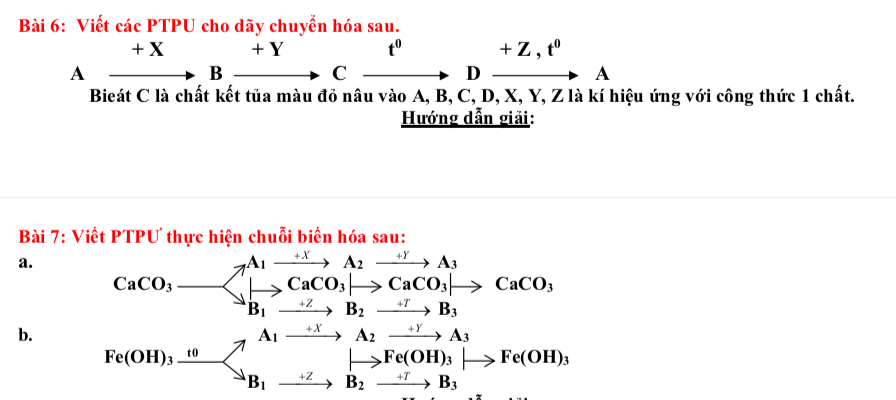
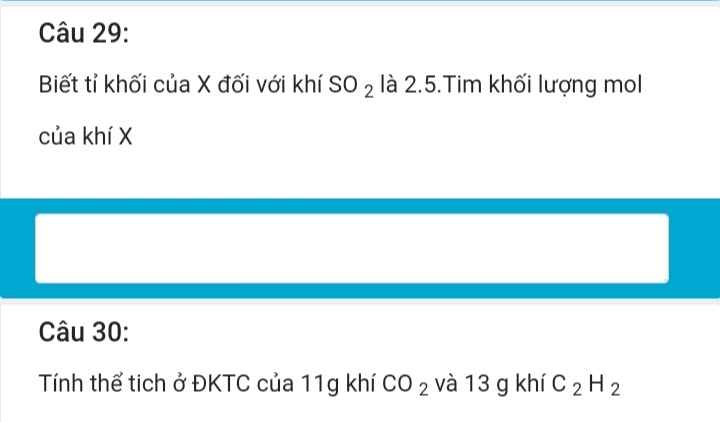
Ta có: \(\left\{{}\begin{matrix}n_{Fe_2O_3}=\dfrac{40\cdot60\%}{160}=0,15\left(mol\right)\\n_{CuO}=\dfrac{40\cdot40\%}{80}=0,2\left(mol\right)\end{matrix}\right.\)
PTHH: \(Fe_2O_3+3H_2\xrightarrow[]{t^o}2Fe+3H_2O\)
0,15____0,45___0,3____0,45 (mol)
\(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
0,2___0,2___0,2___0,2 (mol)
Ta có: \(\left\{{}\begin{matrix}m_{Fe}=0,3\cdot56=16,8\left(g\right)\\m_{Cu}=0,2\cdot64=12,8\left(g\right)\\V_{H_2}=\left(0,2+0,45\right)\cdot22,4=14,56\left(l\right)\end{matrix}\right.\)










 A-Ai cứu mình với...bài nhiều quá...mình không làm nổi...Mấy bạn giúp mình với...
A-Ai cứu mình với...bài nhiều quá...mình không làm nổi...Mấy bạn giúp mình với...