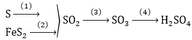Câu 1 :
Gọi $n_{SO_3} = a(mol)$
$SO_3 + H_2O \to H_2SO_4$
Sau khi pha :
$m_{dd} = 80a + 200(gam)$
$m_{H_2SO_4} = 98a + 200.9,8\% = 98a + 19,6(gam)$
Suy ra : $C\%_{H_2SO_4} = \dfrac{98a + 19,6}{200 + 80a}.100\% = 49\%$
$\Rightarrow a = 1,333(mol)$
$m_{SO_3} = 1,333.80 = 106,64(gam)$
Gọi $m_{oleum} = a(gam) ; m_{H_2SO_4} = b(gam)$
Ta có :
Sau khi trộn :
$m_{oleum} = a + b(gam)$
$m_{SO_3} = a.71\% = 0,71a(gam)$
$\Rightarrow \%SO_3 = \dfrac{0,71a}{a + b}.100\% = 62\%$
$\Rightarrow a + b = 0,4402a$
$\Rightarrow \dfrac{a}{b} = \dfrac{1}{1 - 0,4402} = 1,78$
1.
\(m_{H_2SO_4\left(9.8\%\right)}=200\cdot9.8\%=19.6\left(g\right)\)
\(n_{H_2SO_4}=\dfrac{19.6}{98}=0.2\left(mol\right)\)
\(TC:n_{SO_3}=a\left(mol\right)\)
\(SO_3+H_2O\rightarrow H_2SO_4\)
\(a................a\)
\(m_{H_2SO_4\left(tt\right)}=98a\left(g\right)\)
\(m_{H_2SO_4\left(tổng\right)}=19.6+98a\left(g\right)\)
\(m_{dd_{H_2SO_4}}=200+80a\left(g\right)\)
\(C\%_{H_2SO_4}=\dfrac{19.6+98a}{200+80a}\cdot100\%=49\%\)
\(\Rightarrow a=\dfrac{4}{3}\)
\(m_{SO_3}=\dfrac{4}{3}\cdot80=106.67\left(g\right)\)