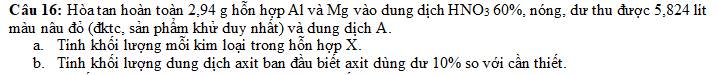a)
Gọi $n_{Al} = a(mol) ; n_{Mg} = b(mol) \Rightarrow 27a + 24b = 2,94(1)$
$n_{NO_2} = \dfrac{5,824}{22,4} = 0,26(mol)$
Bảo toàn electron : $3a + 2b = 0,26(2)$
Từ (1)(2) suy ra : a = 0,02 ; b = 0,1
$m_{Al} = 0,02.27 = 0,54(gam)$
$m_{Mg} = 0,1.24 = 2,4(gam)$
b)
$n_{HNO_3} = 2n_{NO_2} = 0,52(mol)$
$n_{HNO_3\ dư} = 0,52.10\% = 0,052(mol)$
$\Rightarrow n_{HNO_3\ đã\ dùng} = 0,572(mol)$
$\Rightarrow m_{dd\ HNO_3} = \dfrac{0,572.63}{60\%} = 60,06(gam)$