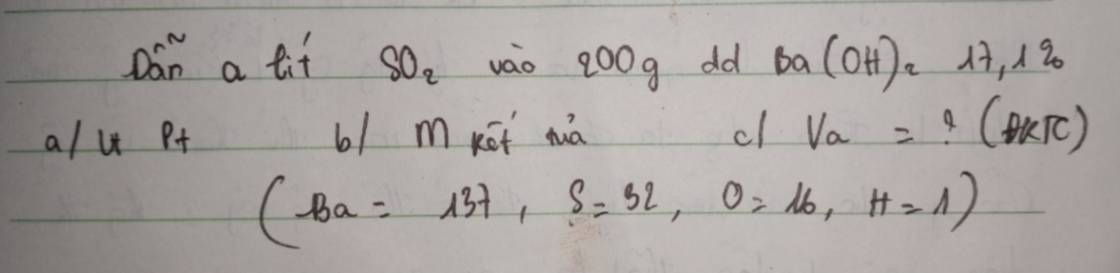a. \(n_{Ba\left(OH\right)_2}=\dfrac{200.17,1\%}{171}=0,2\left(mol\right)\)
PTHH : Ba(OH)2 + SO2 -> BaSO3 \(\downarrow\) + H2O
0,2 0,2 0,2
b. \(m_{BaSO_3}=0.2.217=43,4\left(g\right)\)
c. \(V_{SO_2}=0,2.22,4=4,48\left(l\right)\)
a) Ta có: \(n_{Ba\left(OH\right)_2}=\dfrac{200.17,1\%}{171}=0,2\left(mol\right)\)
PTHH: \(Ba\left(OH\right)_2+SO_2\rightarrow BaSO_3\downarrow+H_2O\)
0,2-------->0,2------->0,2 (mol)
b) mkết tủa = \(m_{BaSO_3}=0,2.217=43,4\left(g\right)\)
c) \(V_a=V_{SO_2}=0,2.22,4=4,48\left(l\right)\)