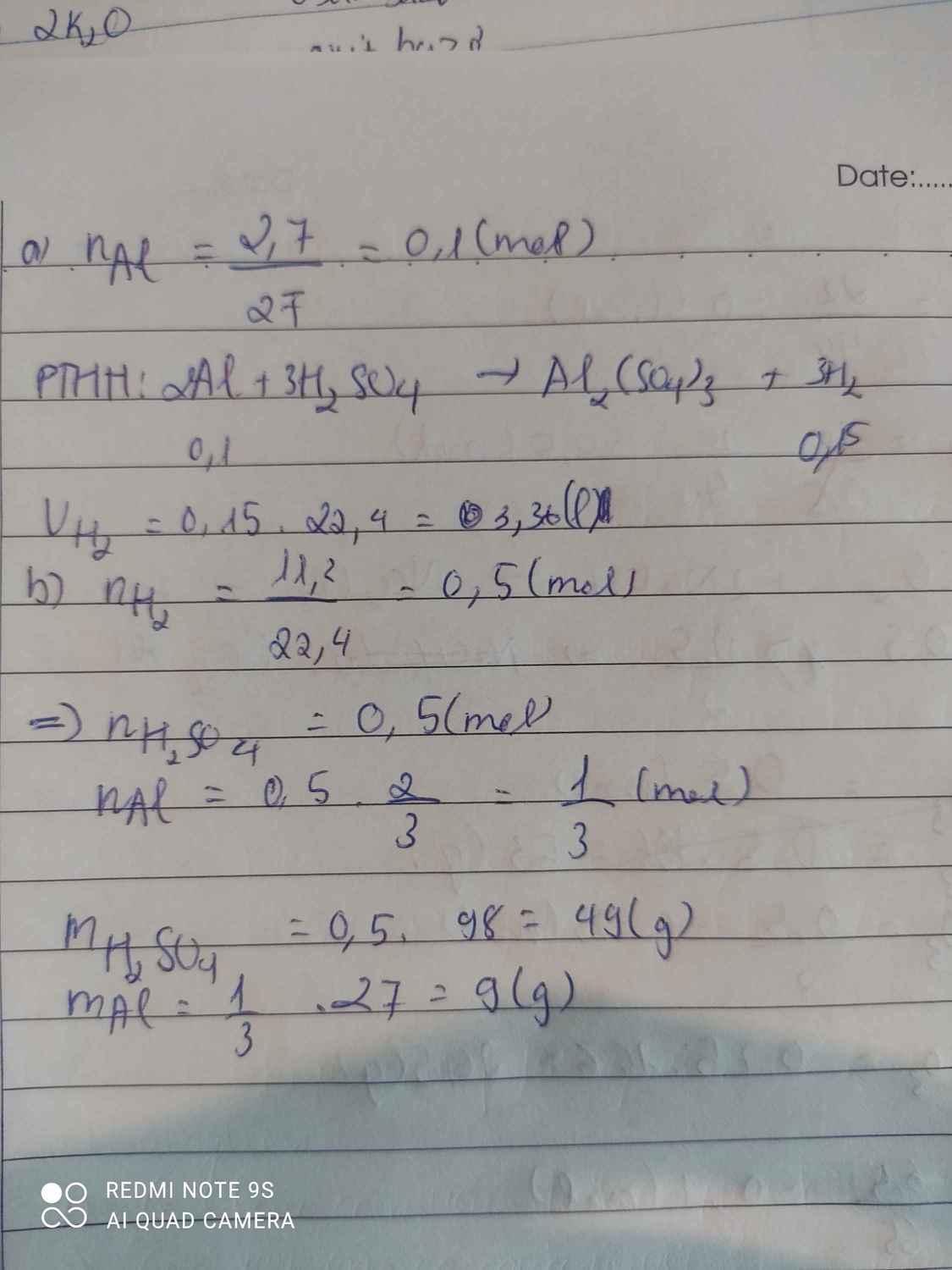Em cần bài 6 em nhỉ?
Bài 6:
\(a,n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ n_{H_2}=\dfrac{3}{2}.n_{Al}=1,5.0,1=0,15\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,15.22,4=3,36\left(l\right)\\ b,n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ n_{Al}=\dfrac{2}{3}.0,5=\dfrac{1}{3}\left(mol\right)\\ \Rightarrow m_{Al}=\dfrac{1}{3}.27=9\left(g\right)\\ n_{H_2SO_4}=n_{H_2}=0,5\left(mol\right)\\ \Rightarrow m_{H_2SO_4}=0,5.98=49\left(g\right)\)