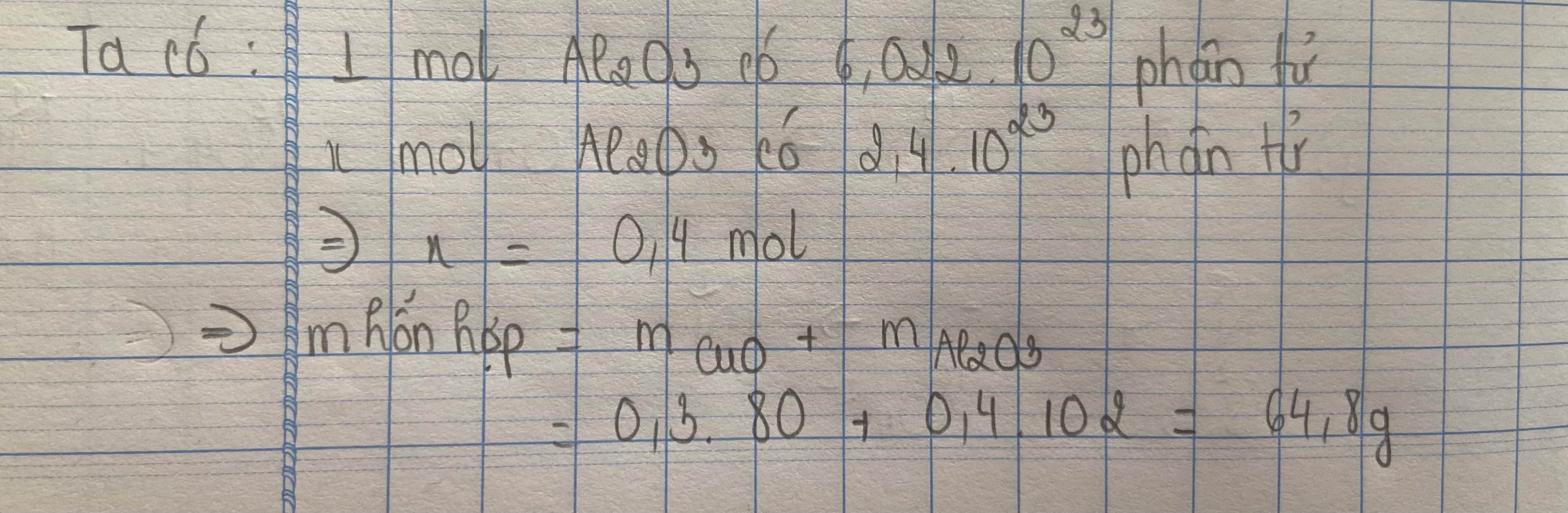\(m_{CuO}=n.M=0,3.80=24\left(g\right)\\ n_{Al_2O_3}=\dfrac{2.4.10^{23}}{6.10^{23}}=0,4\left(mol\right)\\ \Rightarrow m_{Al_2O_3}=n.M=0,4.102=40,8\left(g\right)\\ \Rightarrow m_{hh}=m_{CuO}+m_{Al_2O_3}=24+40,8=64,8\left(g\right)\)
$n_{Al_2O_3} = \dfrac{2.10^{23}}{6.10^{23}} = \dfrac{1}{3}(mol)$
$m_{hỗn\ hợp} = m_{CuO} + m_{Al_2O_3} = 0,3.80 + \dfrac{1}{3}.102 = 58(gam)$