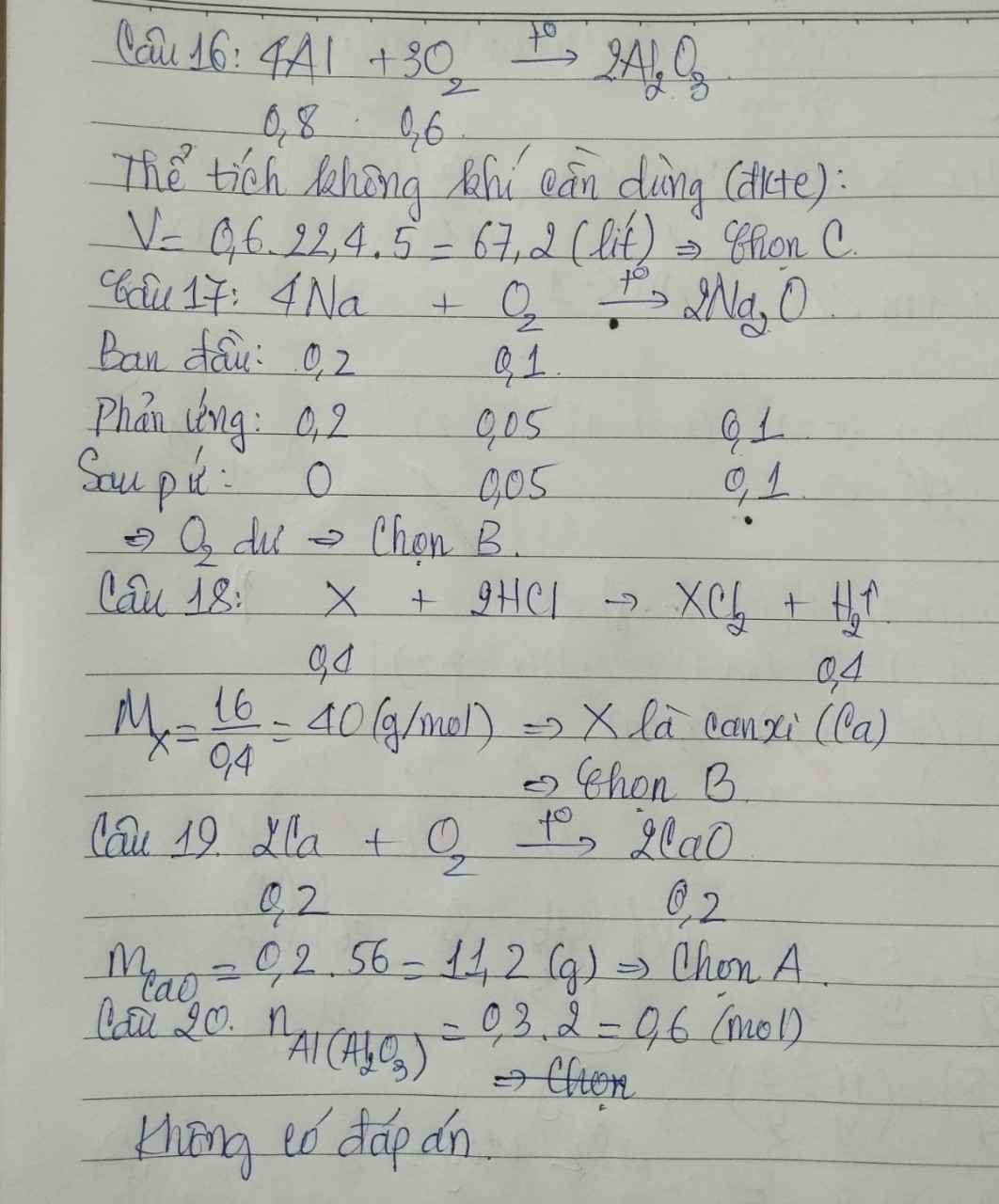Câu 16: C
\(n_{Al}=\dfrac{21,6}{27}=0,8\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,8->0,6
=> VO2 = 0,6.22,4 = 13,44 (l)
=> Vkk =13,44 : 20% = 67,2 (l)
Câu 17: B
\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\); \(n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: 4Na + O2 --to--> 2Na2O
Xét tỉ lệ : \(\dfrac{0,2}{4}< \dfrac{0,1}{1}\) => Na hết, O2 dư
Câu 18: B
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: X + 2HCl --> XCl2 + H2
0,4<-------------------0,4
=> \(M_X=\dfrac{16}{0,4}=40\left(g/mol\right)\)
=> X là Ca
Câu 19: A
\(n_{Ca}=\dfrac{8}{40}=0,2\left(mol\right)\)
PTHH: 2Ca + O2 --to--> 2CaO
0,2---------------->0,2
=> mCaO = 0,2.56 = 11,2 (g)
Câu 20: Không có đáp án thỏa mãn
\(n_{Al}=0,3.2=0,6\left(mol\right)\)
\(16.4Al+3O_2-^{t^o}\rightarrow2Al_2O_3\\ n_{O_2}=\dfrac{3}{4}n_{Al}=0,6\left(mol\right)\\ \Rightarrow V_{O_2}=0,6.22,4=13,44\left(l\right)\\ VìtrongkhôngkhíO_2chiếm20\%\\ \Rightarrow V_{kk}=\dfrac{V_{O_2}}{20\%}=67,2\left(l\right)\\ 17.4Na+O_2-^{t^o}\rightarrow2Na_2O\\n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right);n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ LTL:\dfrac{0,2}{4}< \dfrac{0,1}{1}\\ \Rightarrow O_2dư\\ 18.X+2HCl\rightarrow XCl_2+H_2\\ n_X=n_{H_2}=0,4\left(mol\right)\\ \Rightarrow M_X=\dfrac{16}{0,4}=40\left(Ca\right)\)