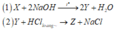a)
\(K_{\left(a\right)}=\dfrac{\left(NH_4^+\right)^2\left(Cu\left(OH\right)_2\right)}{\left(Cu^{2+}\right)\left(NH_3\right)^2\left(H_2O\right)^2}\)
Coi \(\left(Cu\left(OH\right)_2\right)=1\) (chất rắn, được coi là nguyên chất); \(\left(H_2O\right)=1\) (dung môi, trong dung dịch loãng). Vậy:
\(K_{\left(a\right)}=\dfrac{\left(NH_4^+\right)^2}{\left(Cu^{2+}\right)\left(NH_3\right)^2}=\dfrac{\left[NH_4^+\right]^2.f^2_{NH_4^+}}{\left[Cu^{2+}\right].f_{Cu^{2+}}.\left[NH_3\right]^2.f^2_{NH_3}}\)
Ở lực ion thấp, coi gần đúng \(f_i=1\), lúc đó:
\(K_{\left(a\right)}=\dfrac{\left[NH_4^+\right]^2}{\left[Cu^{2+}\right]\left[NH_3\right]^2}=K_C\)
b)
\(K_{\left(a\right)}=\dfrac{\left(Cu\left(NH_3\right)_4^{2+}\right)^2\left(OH^-\right)^4}{\left(Cu\right)^2P_{O_2}\left(NH_3\right)^8\left(H_2O\right)^2}=\dfrac{\left[Cu\left(NH_3\right)_4^{2+}\right]^2\left[OH^-\right]^4f^2_{Cu\left(NH_3\right)_4^{2+}}f^4_{OH^-}}{P_{O_2}\left[NH_3\right]^8f^8_{NH_3}}\)
Coi `(Cu)=1` (chất rắn); \(\left(H_2O\right)=1\) (dung môi). Nếu coi \(f_i=1\) thì
\(K_{\left(a\right)}=\dfrac{\left[Cu\left(NH_3\right)_4^{2+}\right]^2\left[OH^-\right]^4}{P_{O_2}\left[NH_3\right]^8}=K_C\)