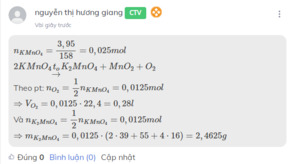
PTHH : \(2KMnO_4\left(t^o\right)->K_2MnO_4+MnO_2+O_2\uparrow\) (1)
Có : \(n_{KMnO_4}=\dfrac{m}{M}=\dfrac{3,95}{158}=0,025\left(mol\right)\)
Từ (1) -> \(n_{O_2}=\dfrac{1}{2}n_{KMnO_4}=0,0125\left(mol\right)\)
-> \(V_{O_2\left(đktc\right)}=n.22,4=0,0125.22,4=0,28\left(l\right)\)
Từ (1) -> \(n_{K_2MnO_4}=\dfrac{1}{2}n_{KMnO_4}=0,0125\left(mol\right)\)
-> \(m_{K_2MnO_4}=n.M=2,4625\left(g\right)\)