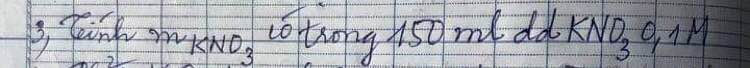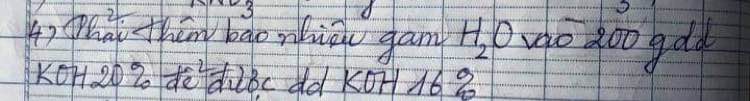\(n_{H_2}=\dfrac{13,44}{22,4}=0,6mol\\ a)2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,4 1,2 0,4 0,6
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\\ b)m_{Al}=0,4.27=10,8g\\ \%m_{Al}=\dfrac{10,8}{21}\cdot100\%=51,43\%\\ \%m_{Al_2O_3}=100\%-51,43\%=48,57\%\\ c)m_{Al_2O_3}=21-10,8=10,2g\\ n_{Al_2O_3}=\dfrac{10,2}{102}=0,1mol\\ Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
0,1 0,6 0,2 0,3
\(n_{HCl}=1,2+0,6=1,8mol\\ m_{HCl}=1,8.36,5=65,7g\\ C_{\%HCl}=\dfrac{65,7}{200}\cdot100\%=32,85\%\\ d)m_{dd}=21+200-0,6.2=219,8g\\ n_{AlCL_3}=0,4+0,2=0,6mol\\ m_{AlCl_3}=0,6.133,5=80,1g\\ C_{\%AlCl_3}=\dfrac{80,1}{219,8}\cdot100\%=36,44\%\)

 mình cần gấp
mình cần gấp



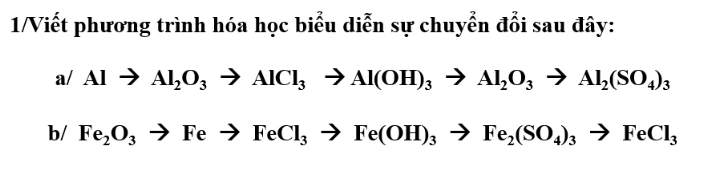



 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ