Em làm bài kiểm tra đúng không em? Nếu làm bài kiểm tra mình tự làm nhé!
\(n_{H_2}=\dfrac{1,8}{2}=0,9\left(mol\right)\\ a,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ b,Đặt:n_{Al}=a\left(mol\right);n_{Fe}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}27a+56b=27,6\\1,5a+b=0,9\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,4\\b=0,3\end{matrix}\right.\\ \Rightarrow m_{Al}=0,4.27=10,8\left(g\right);m_{Fe}=0,3.56=16,8\left(g\right)\\ c,\%m_{Al}=\dfrac{10,8}{27,6}.100\approx39,13\%\Rightarrow\%m_{Fe}\approx60,87\%\\ d,n_{FeSO_4}=n_{Fe}=0,3\left(mol\right);n_{Al_2\left(SO_4\right)_3}=\dfrac{n_{Al}}{2}=\dfrac{0,4}{2}=0,2\left(mol\right)\\ \Rightarrow m_{muối}=0,2.342+0,3.152=114\left(g\right)\\ e,n_{H_2SO_4}=n_{H_2}=0,9\left(mol\right)\\ \Rightarrow m_{H_2SO_4}=0,9.98=88,2\left(g\right)\)














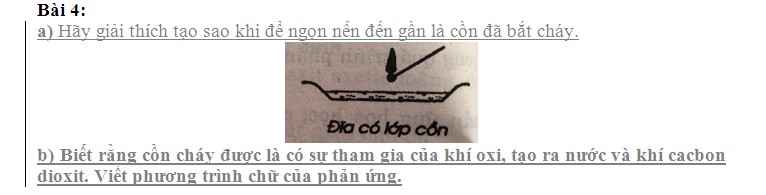 mấy anh chị giải chi tiết giùm em,em cần lời giải chi tiết ạ em xin cảm ơn
mấy anh chị giải chi tiết giùm em,em cần lời giải chi tiết ạ em xin cảm ơn

