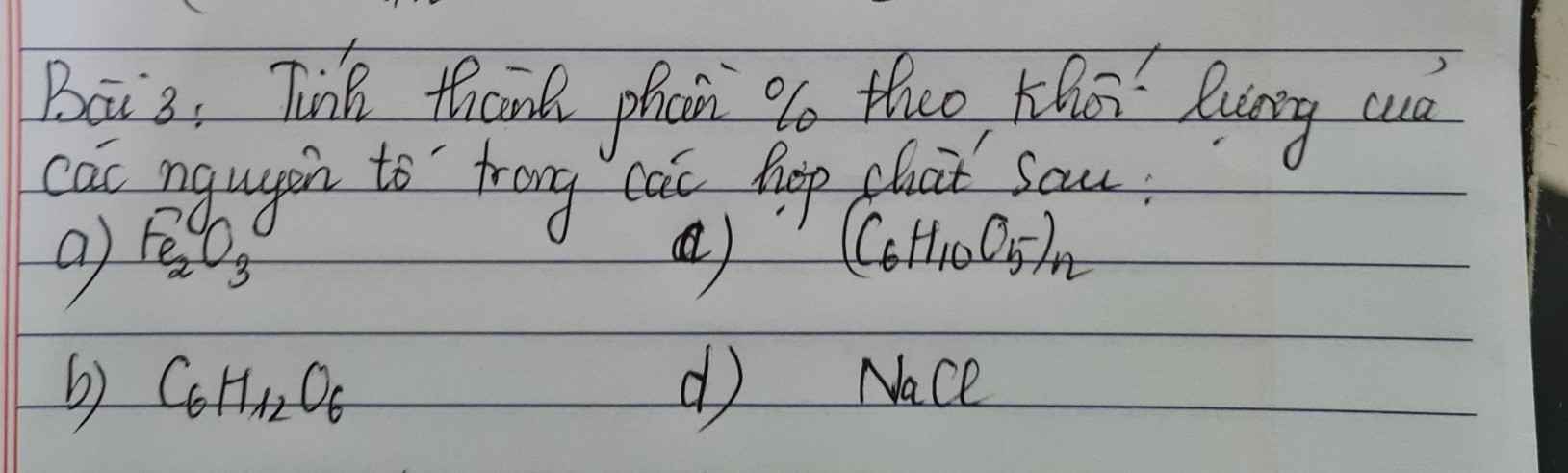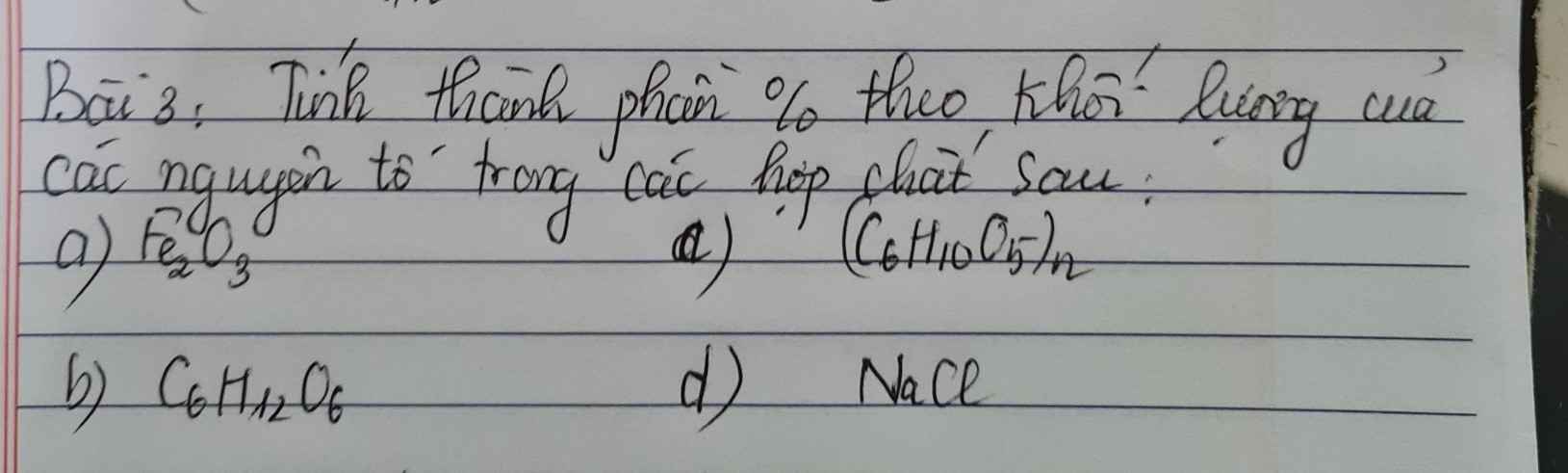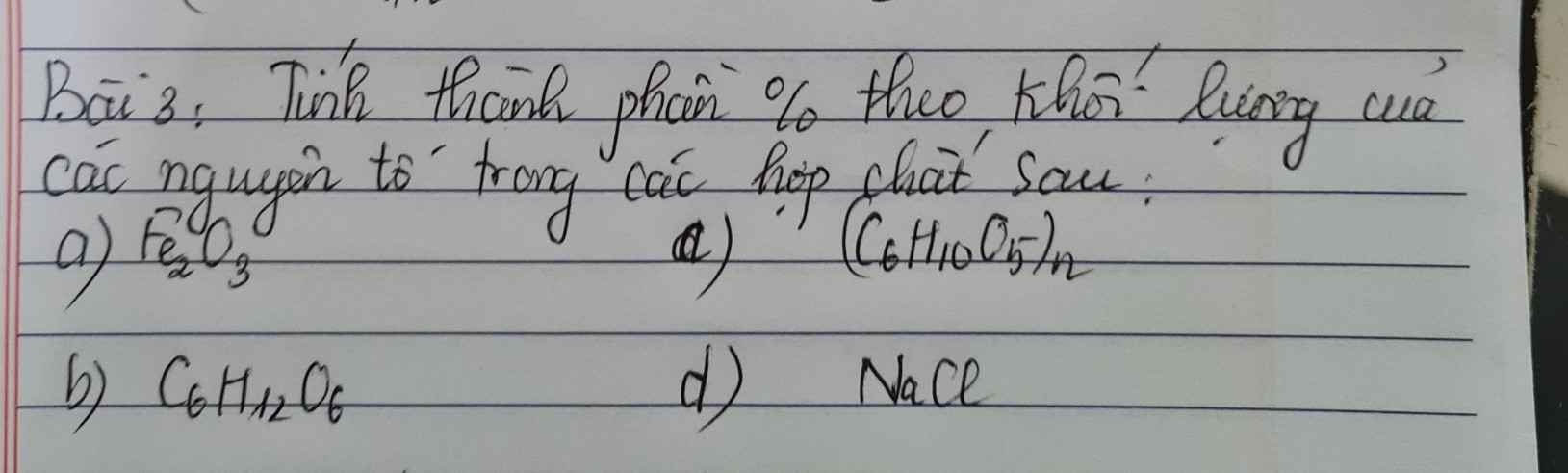Bài 1.
\(n_{Zn}=\dfrac{16,25}{65}=0,25mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,25 0,5 0,25
\(m_{HCl}=0,5\cdot36,5=18,25\left(g\right)\)
\(V_{H_2}=0,25\cdot22,4=5,6\left(l\right)\)
Bài 2.
\(n_{AlCl_3}=\dfrac{13,35}{133,5}=0,1mol\)
\(2Al+3Cl_2\underrightarrow{t^o}2AlCl_3\)
0,1 0,15 0,1
\(m_{Al}=0,1\cdot27=2,7\left(g\right)\)
\(V_{Cl_2}=0,15\cdot22,4=3,36\left(l\right)\)
Bài 3.
\(n_{Cu}=\dfrac{1,6}{64}=0,025mol\)
\(CuO+H_2\rightarrow Cu+H_2O\)
0,025 0,025
\(m_{CuO}=0,025\cdot80=2\left(g\right)\)