a) \(n_{Al_2O_3}=0,75\left(mol\right)n_{H_2SO_4}=0,45\left(mol\right)\)
\(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\)
Lập tỉ lệ : \(\dfrac{0,75}{1}>\dfrac{0,45}{3}\) => Sau phản ứng Al2O3 dư
=>\(m_{Al_2O_3\left(dư\right)}=\left(0,75-\dfrac{0,45}{3}\right).102=61,2\left(g\right)\)
b) \(m_{ddsauphanung}=15,3+735=750,3\left(g\right)\)
=> \(C\%_{Al_2\left(SO_4\right)_3}=\dfrac{0,15.342}{750,3}.100=6,84\%\)

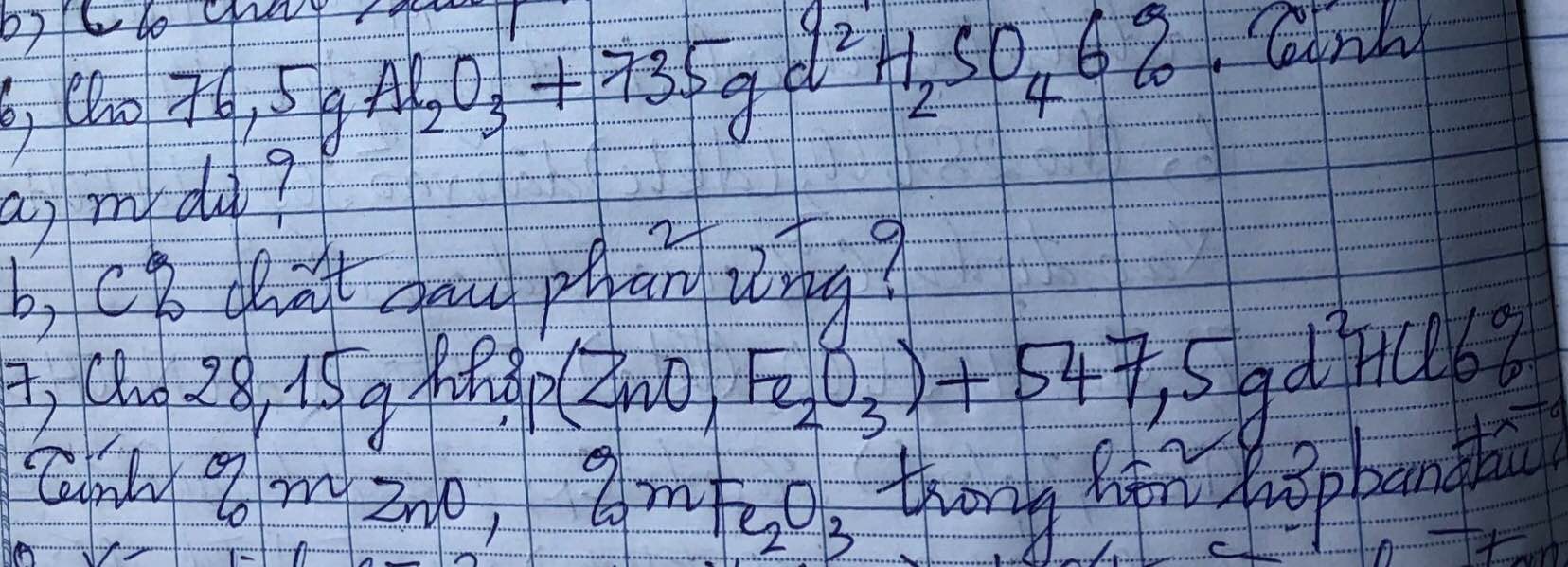








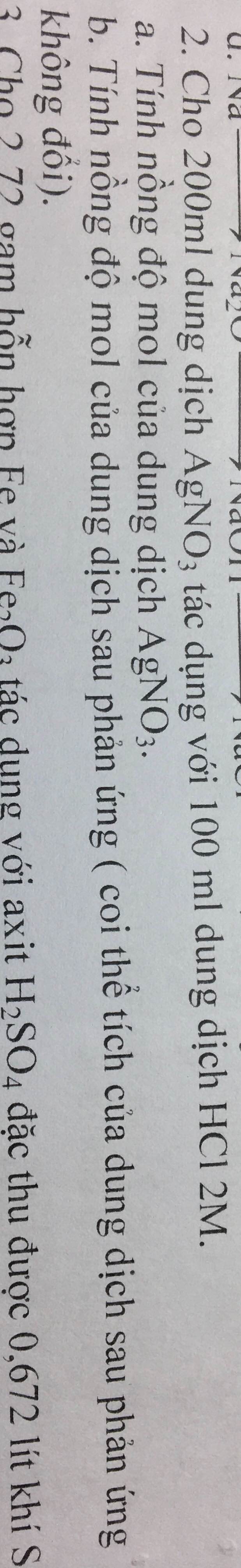

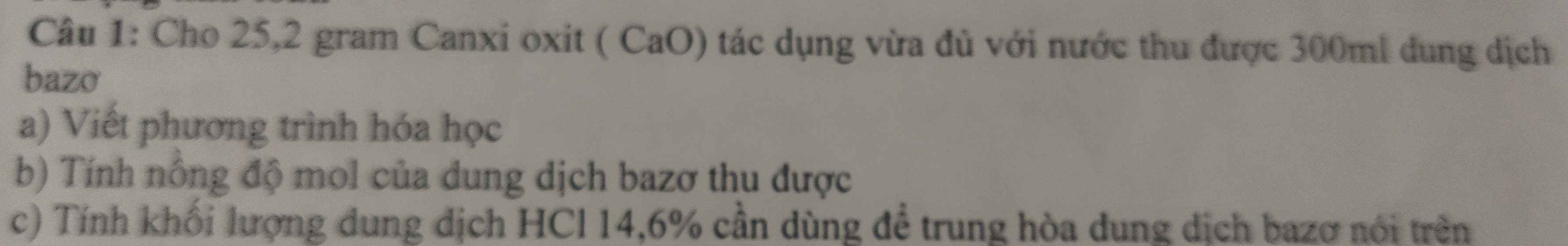
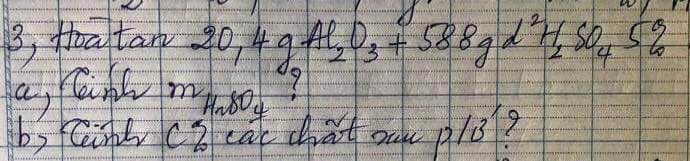





 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ