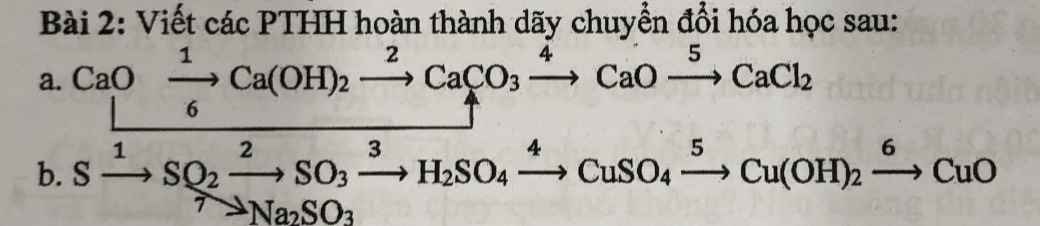
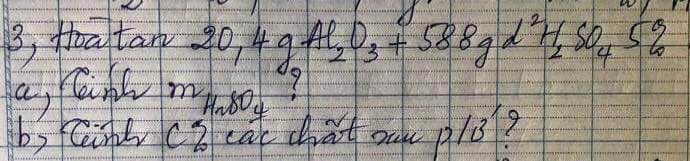Bài 4 :
\(n_{H2}=\dfrac{V_{H2}}{22,4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2|\)
2 3 1 3
0,1 0,15 0,05 0,15
a) \(n_{Al}=\dfrac{0,15.2}{3}=0,1\left(mol\right)\)
⇒ \(m_{Al}=n_{Al}.M_{Al}\)
= 0,1 . 27
= 2,7 (g)
\(m_{Cu}=10-2,7=7,3\left(g\right)\)
0/0Al = \(\dfrac{m_{Al}.100}{m_{hh}}=\dfrac{2,7.100}{10}=27\)0/0
0/0Cu = \(\dfrac{m_{Cu}.100}{m_{hh}}=\dfrac{7,3.100}{10}=13\)0/0
b) \(n_{Al2\left(SO4\right)3}=\dfrac{0,15.1}{3}=0,05\left(mol\right)\)
⇒ \(m_{Al2\left(SO4\right)3}=n_{Al2\left(SO4\right)3.}M_{Al2\left(SO4\right)3}\)
= 0,05 . 342
= 17,1 (g)
\(n_{H2SO4}=\dfrac{0,1.3}{2}=0,15\left(mol\right)\)
⇒ \(m_{H2SO4}=n_{H2SO4}.M_{H2SO4}\)
= 0,15 .98
= 14,7 (g)
\(C_{H2SO4}=\dfrac{m_{ct}.100}{m_{dd}}\Rightarrow m_{dd}=\dfrac{m_{ct}.100}{C}=\)\(\dfrac{14,7.100}{15}=98\left(g\right)\)
mdung dịch sau phản ứng = (mAl + mCu) + mH2SO4 - mH2
= 10 + 98 - (0,15 . 2)
=107,7 (g)
\(C_{Al2\left(SO4\right)3}=\dfrac{m_{ct}.100}{m_{dd}}=\dfrac{17,1.100}{107,7}=15,88\)0/0
Chúc bạn học tốt