a)
nếu như tính khối lượng MOL thì Mg=24(g) luôn
b)
\(n_{Al}=\dfrac{m}{M}=\dfrac{540}{27}=20\left(mol\right)\\ N_{Al}=20\cdot6\cdot10^{23}=1,2\cdot10^{25}\left(nguyentuAl\right)\)
c)
\(n_{CO_2}=\dfrac{1,5\cdot10^{23}}{6\cdot10^{23}}=0,25\left(mol\right)\\ m_{CO_2}=n\cdot M=0,25\cdot44=11\left(g\right)\)













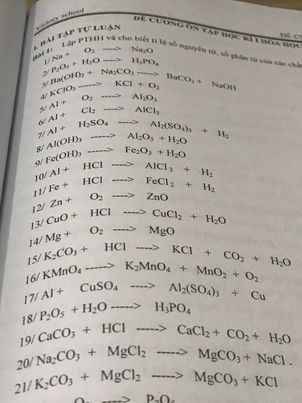




 Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )


 giúp mình với mình chỉ cần 2 câu 8 với câu 9 thôi nha
giúp mình với mình chỉ cần 2 câu 8 với câu 9 thôi nha 
