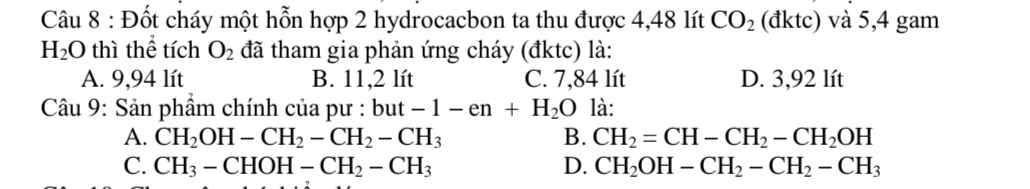
Câu 9:
\(94n_{C_6H_5OH}+46n_{C_2H_5OH}=13,04\left(g\right)\left(1\right)\)
PT: \(C_6H_5OH+Na\rightarrow C_6H_5ONa+\dfrac{1}{2}H_2\)
\(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{C_6H_5OH}+\dfrac{1}{2}n_{C_2H_5OH}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{C_6H_5OH}=0,08\left(mol\right)\\n_{C_2H_5OH}=0,12\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\%n_{C_2H_5OH}=\dfrac{0,12}{0,12+0,08}.100\%=60\%\)
Đáp án: B
Câu 31:
Ta có: m tinh bột = 1000.80% = 800 (kg) \(\Rightarrow n_{\left(C_6H_{10}O_5\right)_n}=\dfrac{800}{162n}=\dfrac{400}{81n}\left(kmol\right)\)
PT: \(\left(C_6H_{10}O_5\right)_n+nH_2O\underrightarrow{H^+,t^o}nC_6H_{12}O_6\)
\(C_6H_{12}O_6\underrightarrow{t^o,xt}2C_2H_5OH+2CO_2\)
\(\Rightarrow n_{C_2H_5OH\left(LT\right)}=2n_{C_6H_{12}O_6}=2n.n_{\left(C_6H_{10}O_5\right)_n}=\dfrac{800}{81}\left(kmol\right)\)
Mà: H = 70% \(\Rightarrow n_{C_2H_5OH\left(TT\right)}=\dfrac{800}{81}.70\%=\dfrac{560}{81}\left(kmol\right)\)
\(\Rightarrow m_{C_2H_5OH\left(TT\right)}=\dfrac{560}{81}.46=\dfrac{25760}{81}\left(kg\right)\)
\(\Rightarrow V_{C_2H_5OH}=\dfrac{\dfrac{25760}{81}}{0,8}=\dfrac{32200}{81}\left(l\right)\)
\(\Rightarrow V_{C_2H_5OH\left(60^o\right)}=\dfrac{\dfrac{32200}{81}}{0,6}\approx662,55\left(l\right)\)
Đáp án: A