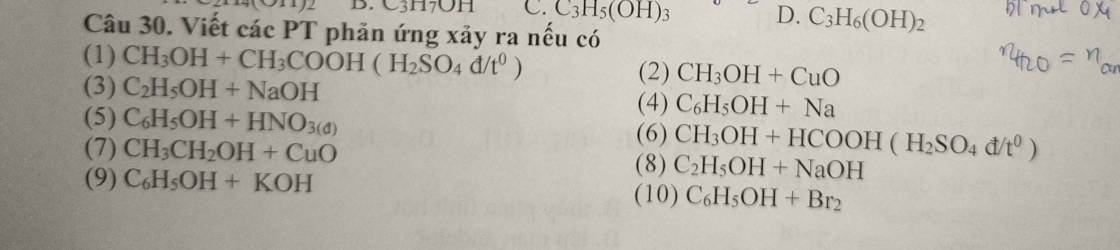$(1) CH_3COOH + CH_3OH \buildrel{{H_2SO_{4\ đ},t^o}}\over\rightleftharpoons CH_3COOCH_3 + H_2O$
$(2) CH_3OH + CuO \xrightarrow{t^o} HCHO + Cu + H_2O$
$(3)$ Không phản ứng
$(4) C_6H_5OH + Na \to C_6H_5ONa + \dfrac{1}{2}H_2$
$(5) C_6H_5OH + 3HNO_{3\ đ} \to C_6H_2(NO_3)_3OH + 3H_2O$
$(6) CH_3OH + HCOOH \buildrel{{H_2SO_{4\ đ},t^o}}\over\rightleftharpoons HCOOCH_3 + H_2O$
$(7) CH_3CH_2OH + CuO \xrightarrow{t^o} CH_3CHO + Cu + H_2O$
$(8) Không phản ứng
$(9) C_6H_5OH + KOH \to C_6H_5OK + H_2O$
$(10) C_6H_5OH + 3Br_2 \to C_6H_2Br_3OH + 3HBr$