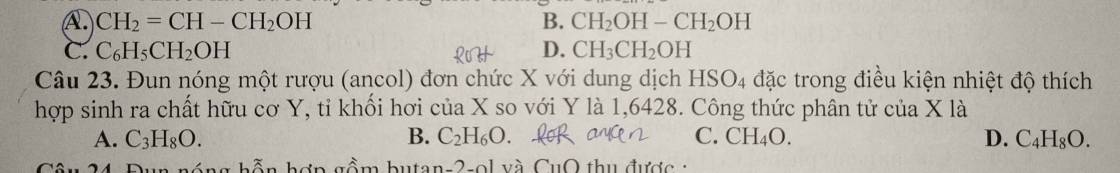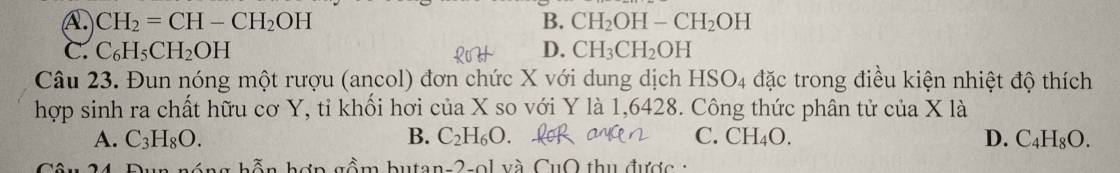1/ Gọi CTTQ hai ankan là $C_nH_{2n+2}$
$C_nH_{2n+2} + \dfrac{3n+1}{2}O_2 \xrightarrow{t^o} nCO_2 + (n + 1)H_2O$
$n_{CO_2} = \dfrac{4,48}{22,4} = 0,2(mol)$
$n_{ankan} = \dfrac{1}{n}.n_{CO_2} = \dfrac{0,2}{n}(mol)$
$\Rightarrow \dfrac{0,2}{n}.(14n + 2) = 2,89$
$\Rightarrow n = 4,4$
Vậy hai ankan là $C_4H_{10}$ và $C_5H_{12}$
2/ $n_{C_4H_{10}} = a(mol) ; n_{C_5H_{12}} = b(mol)$
$\Rightarrow 58a + 72b = 2,89$
$n_{CO_2} = 4a + 5b = 0,2$
$\Rightarrow a = 0,025 ; b = 0,02$
$\%m_{C_4H_{10}} = \dfrac{0,025.58}{2,89}.100\% = 50,2\%$

 Giúp mình cau 23
Giúp mình cau 23