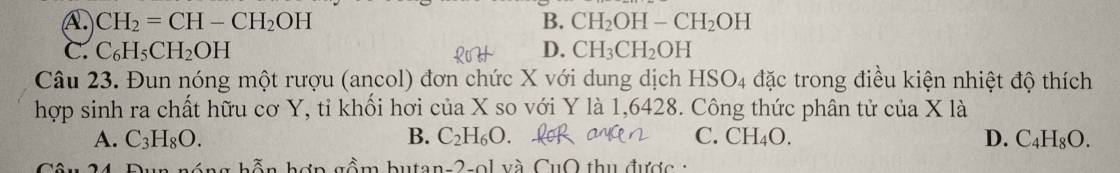\(n_{RS_2}=\dfrac{4,8}{M_R+64}=a\left(mol\right)\)
PTHH: \(4RS_2+11O_2\xrightarrow[]{t^o}2R_2O_3+8SO_2\)
a-------------------->0,5a
\(R_2O_3+6HNO_3\rightarrow2R\left(NO_3\right)_3+3H_2O\)
0,5a---->3a---------->a
=> \(m_{ddHNO_3}=\dfrac{3a.63}{9\%}=2100a\left(g\right)\)
=> \(m_{ddspư}=0,5a.\left(2M_R+48\right)+2100=M_R+2124a\left(g\right)\)
=> \(C\%_{R\left(NO_3\right)_3}=\dfrac{M_R+186a}{M_R+2124a}.100\%=11,1\%\)
=> \(M_R=56\left(g/mol\right)\)
=> R là Fe
=> \(a=\dfrac{4,8}{120}=0,04\left(mol\right)\)
=> \(m_{ddHNO_3}=0,04.2100=84\left(g\right)\)
=> Chọn C