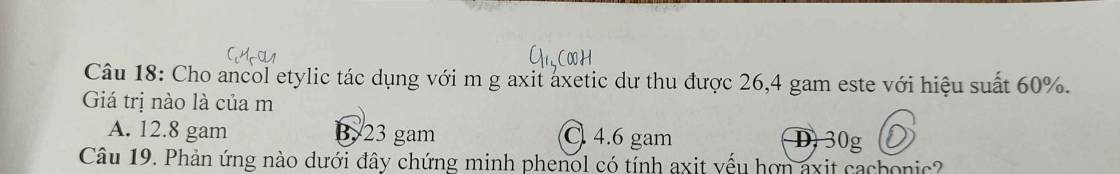\(n_{CH_3COOC_2H_5}=\dfrac{26,4}{88}=0,3\left(mol\right)\)
\(C_2H_5OH+CH_3COOH\xrightarrow[]{H_2SO_4\left(\text{đặc}\right),t^o}CH_3COOC_2H_5\)
\(n_{C_2H_5OH}=n_{CH_3COOC_2H_5}=0,3\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH}=\dfrac{0,3.46}{60\%}=23\left(g\right)\)
→ Chọn B
Đúng 2
Bình luận (0)