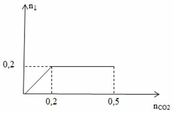
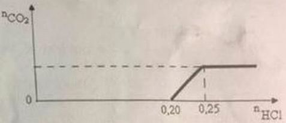
\(n_{HCl}=0.3\left(mol\right)\)
\(n_{K_2CO_3}=0.12\cdot0.6=0.072\left(mol\right)\)
\(n_{NaHCO_3}=0.12\left(mol\right)\)
\(K_2CO_3+HCl\rightarrow KHCO_3+KCl\)
\(0.072......0.072..........0.072\)
\(KHCO_3+HCl\rightarrow KCl+CO_2+H_2O\)
\(0.072.......0.072.....................0.072\)
\(n_{HCl\left(cl\right)}=0.3-0.072-0.072=0.156\left(mol\right)\)
\(NaHCO_3+HCl\rightarrow NaCl+CO_2+H_2O\)
\(0.12............0.12..................0.12\)
\(\Rightarrow HCldư\)
\(n_{CO_2}=0.12+0.072=0.192\left(mol\right)\)