\(a.Zn+2HCl\rightarrow ZnCl_2+_2\)
\(b.n_{H_2}=\dfrac{4.958}{24,79}=0.2\left(mol\right)\)
\(n_{HCl}=2n_{H_2}=2\cdot0.2=0.4\left(mol\right)\)
\(m_{HCl}=0.4\cdot36.5=14.6\left(g\right)\)
\(c.n_{ZnCl_2}=n_{H_2}=0.2\left(mol\right)\)
\(c.m_{ZnCl_2}=0.2\cdot136=27.2\left(g\right)\)
\(d.H=80\%\Rightarrow m_{HCl}=14.6\cdot80\%=11.68\left(g\right)\)
\(n_{Zn}=80\%\cdot n_{H_2}=80\%\cdot0.2=0.16\left(mol\right)\)
\(n_{Zn}=0.16\cdot65=10.4\left(g\right)\)




















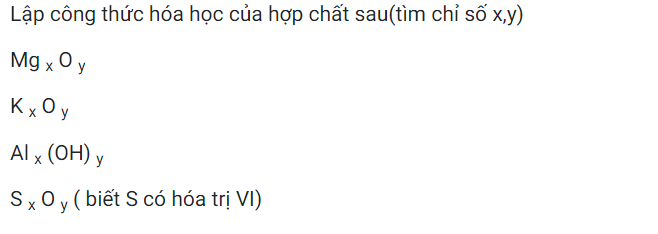


 Ai giúp em với ạ em đang gấp:<<
Ai giúp em với ạ em đang gấp:<<