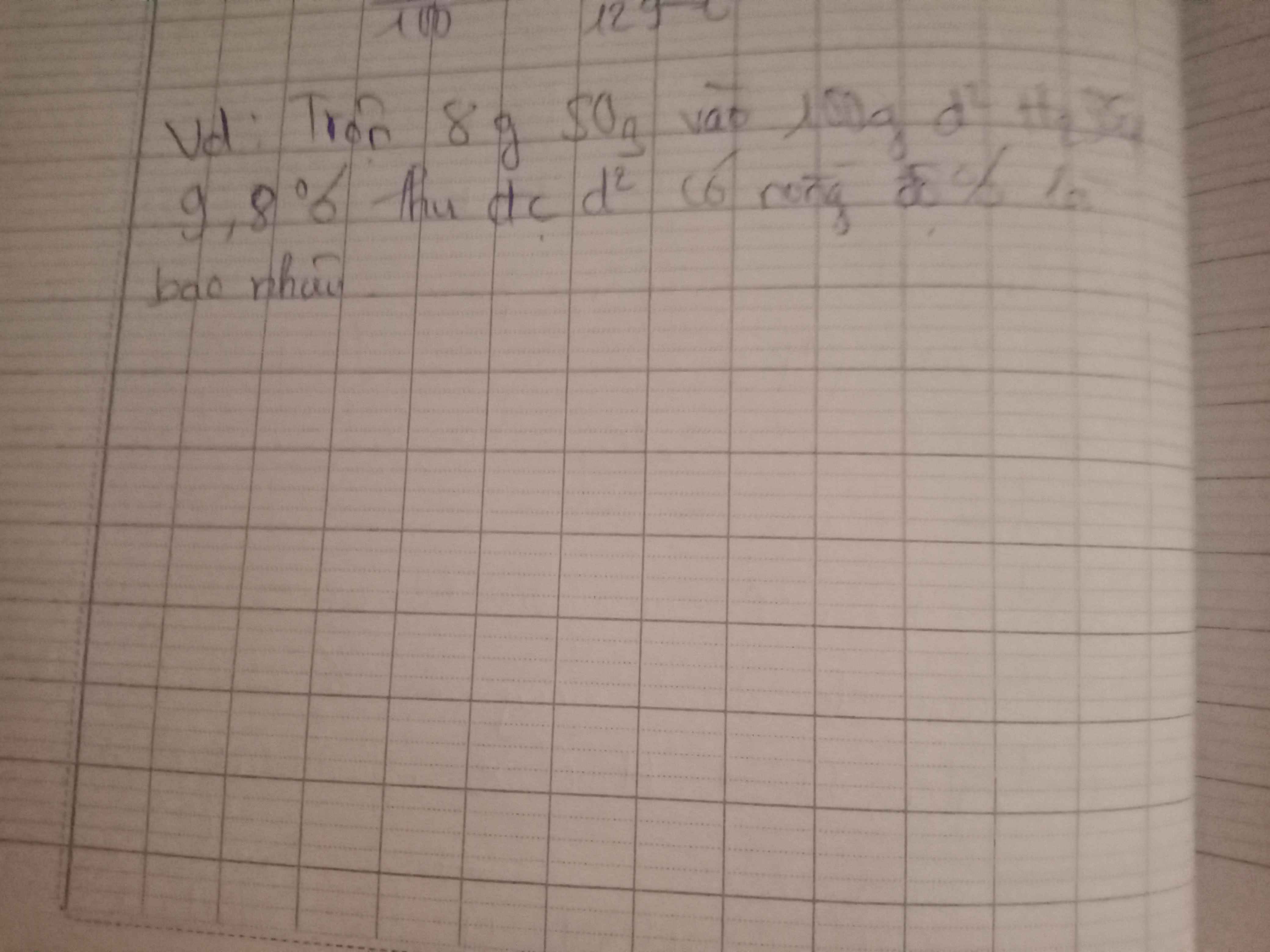
Câu 5 :
\(n_{ZnO}=\dfrac{32,4}{81}=0,4\left(mol\right)\)
a) Pt : \(ZnO+H_2SO_4\rightarrow ZnSO_4+H_2O|\)
0,4 0,4
\(m_{H2SO4}=0,4.98=39,2\left(g\right)\)
\(m_{ddH2SO4}=\dfrac{39,2.100}{9,8\%}=400\left(g\right)\)
c) \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O|\)
0,8 0,4
\(m_{ddNaOH}=\dfrac{\left(0,8.40\right).100}{20\%}=160\left(g\right)\)
\(V_{ddNaOH}=\dfrac{160}{1,11}=144,14\left(ml\right)\)
Chúc bạn học tốt