\(n_{Al}=\dfrac{3,78}{27}=0,14mol\)
\(n_{H_2SO_4}=\dfrac{24,5}{98}=0,25mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,14 0,25 0 0
0,14 0,21 0,07 0,21
0 0,04 0,07 0,21
a)Sau phản ứng, axit sunfuric còn dư và dư:
\(m_{H_2SO_4dư}=0,04\cdot98=3,92g\)
b)\(V_{H_2}=0,21\cdot22,4=4,704l\)
c)\(n_{CuO}=\dfrac{12}{80}=0,15mol\)
\(CuO+H_2\rightarrow Cu+H_2O\)
0,15 0,21 0,15
\(m_{Cu}=0,15\cdot64=9,6g\)
\(n_{Al}=\dfrac{3,78}{27}=0,14\left(mol\right)\\ n_{H_2SO_4}=\dfrac{24,5}{98}=0,25\left(mol\right)\)
\(pthh:2Al+6H_2SO_4->Al_2\left(SO_4\right)_3+3H_2\)
LTL : \(\dfrac{0,14}{2}>\dfrac{0,25}{6}\)
=> Al dư H2SO4 hết
theo pthh : \(n_{Al\left(p\text{ư}\right)}=\dfrac{1}{3}n_{H_2SO_4}=\dfrac{1}{12}\left(mol\right)\)
=> \(m_{Al\left(d\right)}=\left(0,14-\dfrac{1}{12}\right).27=1,53\left(g\right)\)
theo pthh : \(n_{H_2}=n_{H_2SO_4}=0,25\left(mol\right)\)
=> \(V_{H_2}=0,25.22,4=5,6\left(L\right)\)
\(n_{CuO}=\dfrac{12}{80}=0,15\left(mol\right)\\ pthh:CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
LTL :\(\dfrac{0,15}{1}< \dfrac{0,25}{1}\)
=> H2 dư , CuO hết
theo pthh : nCu = nCuO = 0,15 (mol)
=> \(m_{Cu}=0,15.64=9,6\left(g\right)\)











 Ai giúp em với ạ em đang gấp:<<
Ai giúp em với ạ em đang gấp:<<


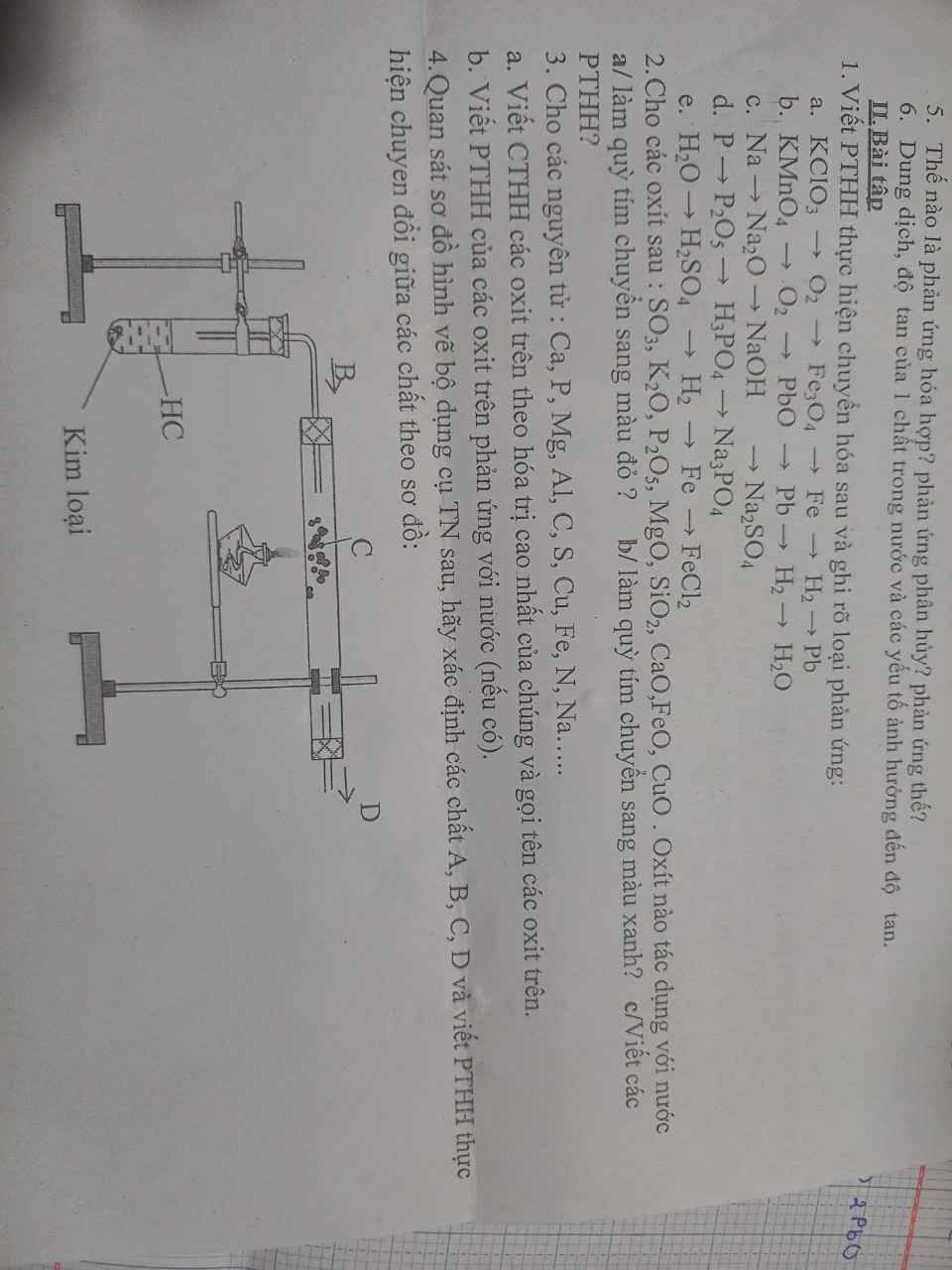 mn giải giúp em với ạ em đag cần gấp , em cảm ơn
mn giải giúp em với ạ em đag cần gấp , em cảm ơn


