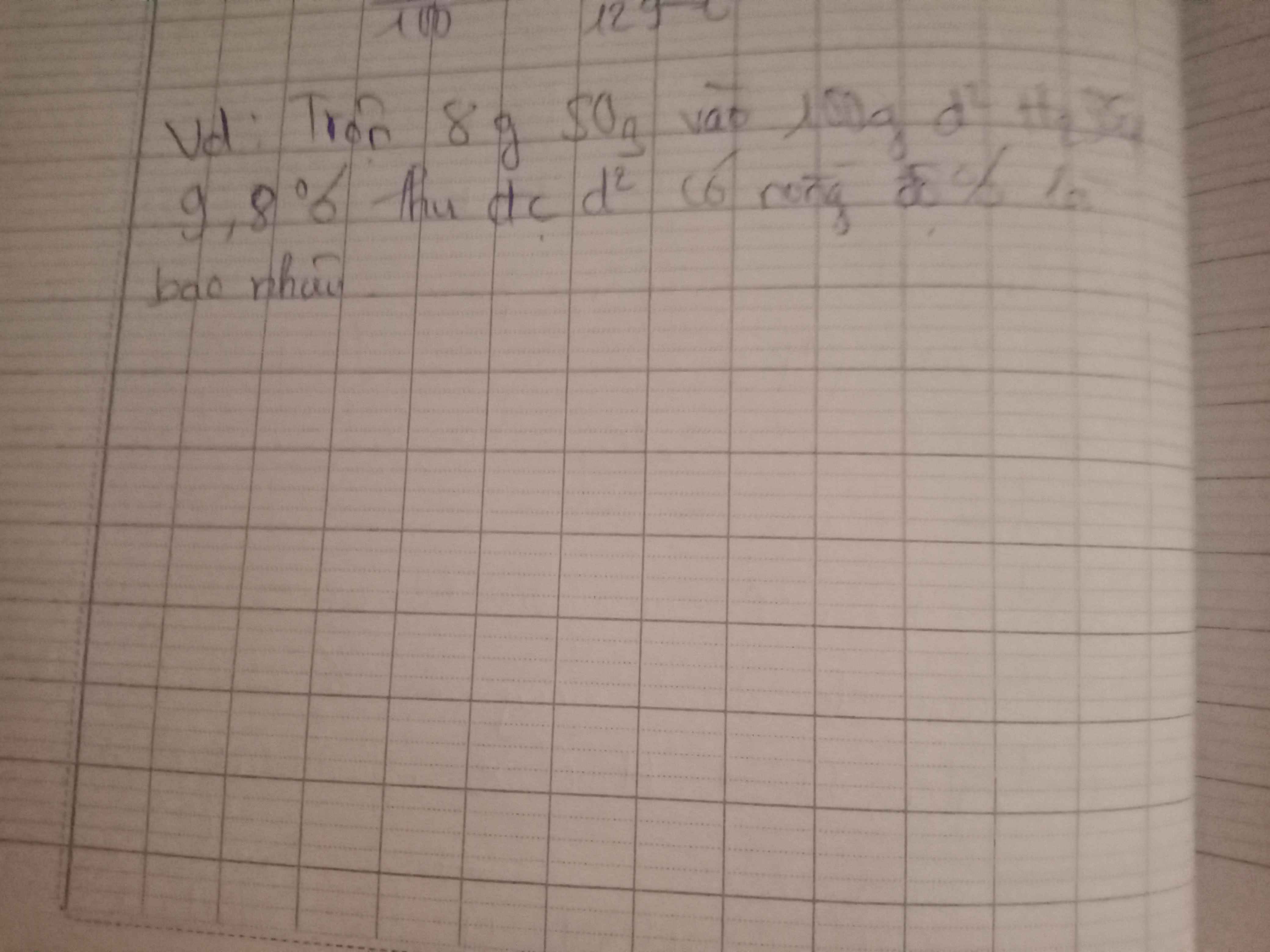
3)
a) \(m_{AgNO_3}=170.40\%=68\left(g\right)\Rightarrow n_{AgNO_3}=\dfrac{68}{170}=0,4\left(mol\right)\)
\(n_{AgCl}=\dfrac{45,92}{143,5}=0,32\left(mol\right)\)
PTHH: \(AgNO_3+HCl\rightarrow AgCl\downarrow+HNO_3\)
0,32<-----0,32<---0,32
=> \(H=\dfrac{0,32}{0,4}.100\%=80\%\)
b) PTHH: \(AgNO_3+HCl\rightarrow AgCl\downarrow+HNO_3\)
0,4----->0,4------->0,4----->0,4
=> \(m_{dd\text{ }sau\text{ }pư}=170+200-0,4.143,5=312,6\left(g\right)\)
=> \(C\%_{HNO_3}=\dfrac{0,4.63}{312,6}.100\%=8,06\%\)