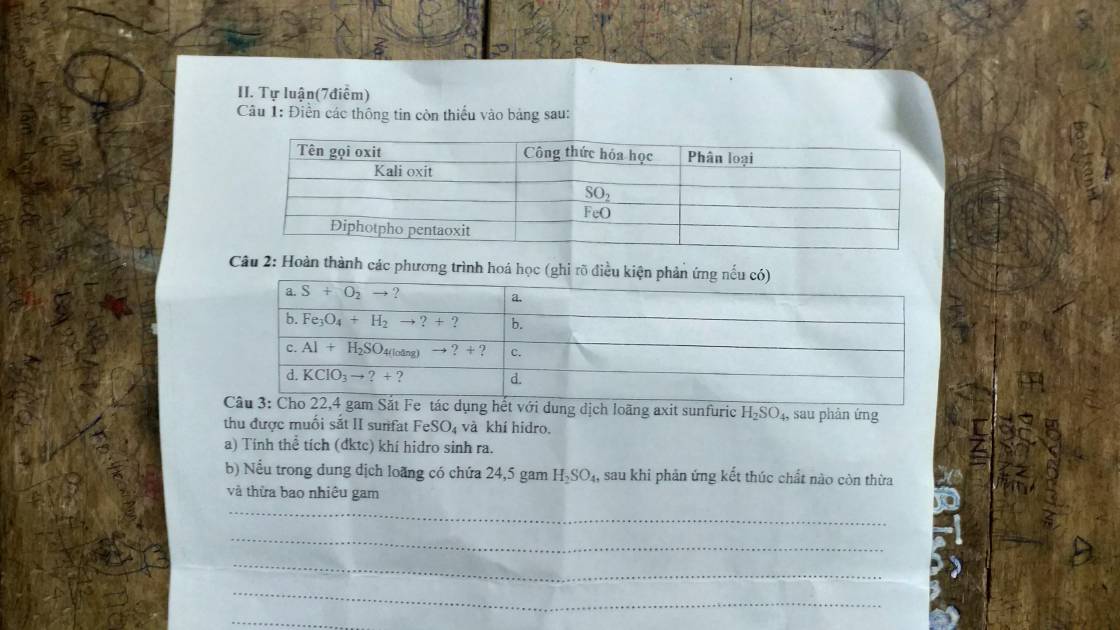
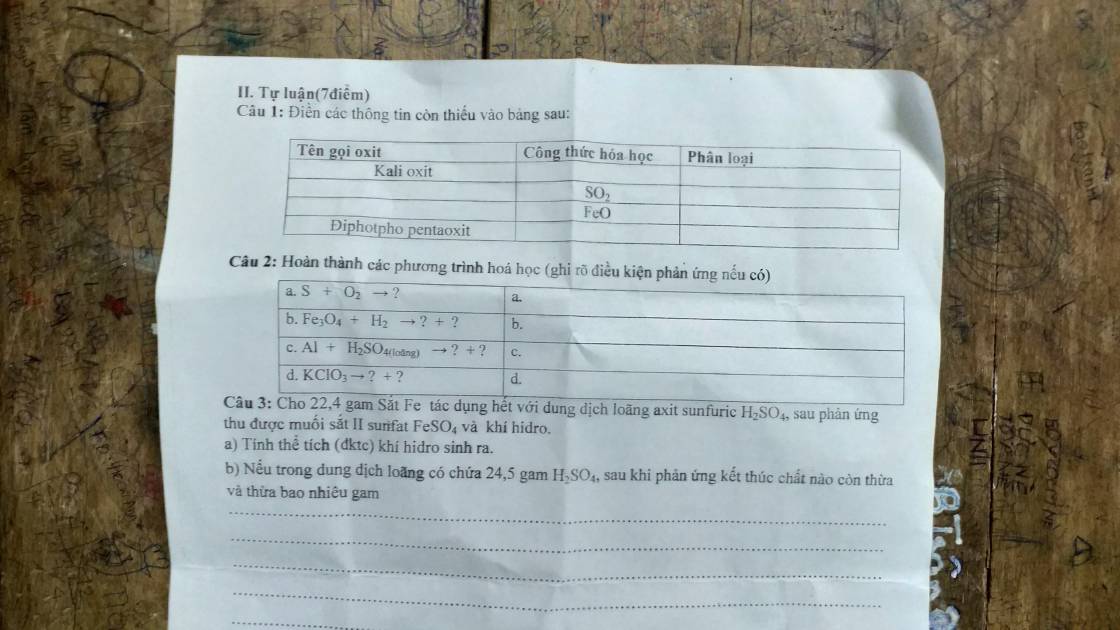
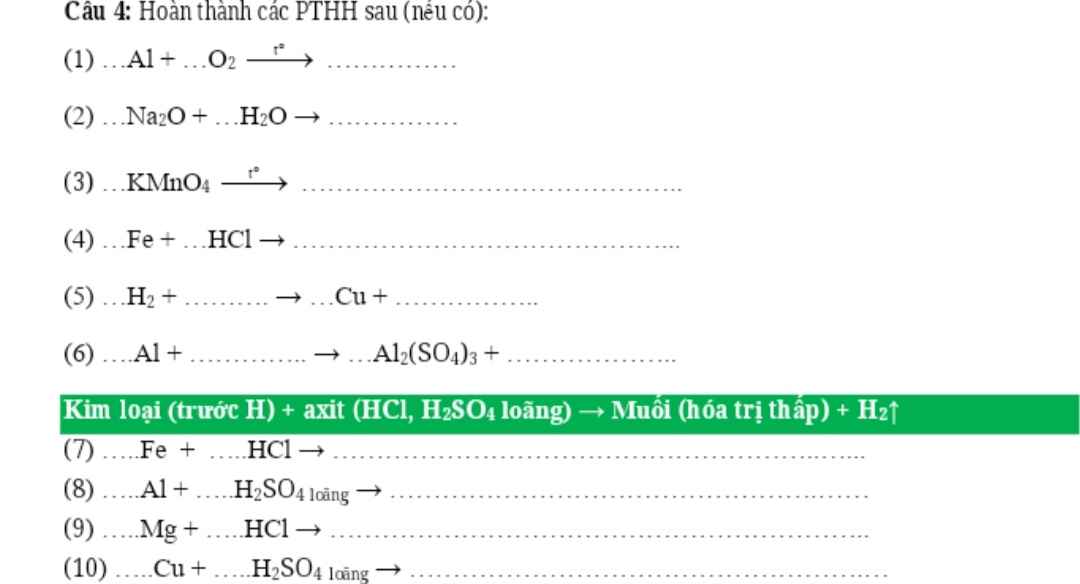
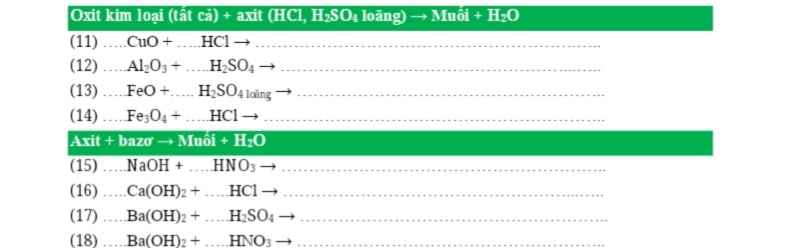
Câu 1:
Kali oxit: K2O: oxit bazo
Lưu huỳnh dioxit: SO2: oxit axit
Sắt (II) oxit: FeO: oxit bazo
Điphotpho pentaoxit: P2O5: oxit axit
Câu 2:
a, \(S+O_2\underrightarrow{t^o}SO_2\)
b, \(Fe_3O_4+4H_2\underrightarrow{t^o}3Fe+4H_2O\)
c, \(2Al+3H_2SO_{4\left(l\right)}\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
d, \(2KClO_3\underrightarrow{t^o,MnO_2}2KCl+3O_2\)