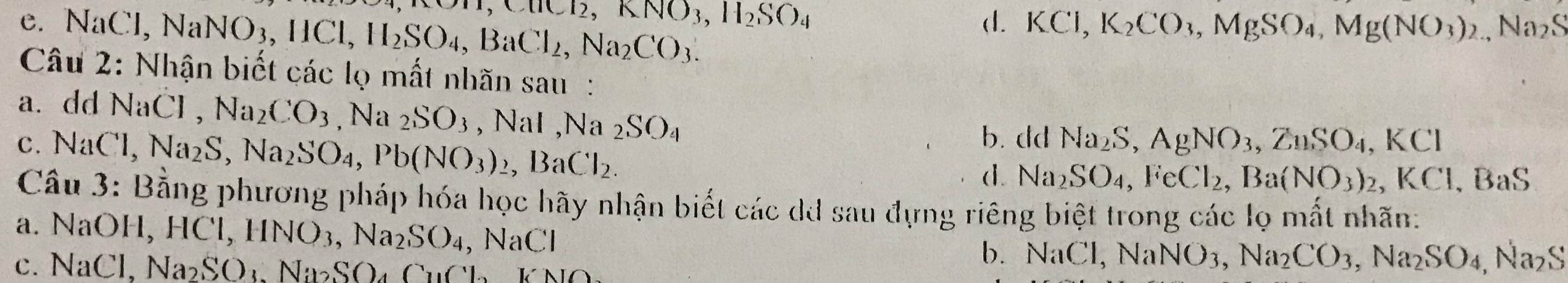\(n_{Al}=\dfrac{2,97}{27}=0,11mol\)
\(n_S=\dfrac{4,08}{32}=0,1275mol\)
\(2Al+3S\rightarrow\left(t^o\right)Al_2S_3\)
\(\dfrac{0,11}{2}\) > \(\dfrac{0,1275}{3}\) ( mol )
0,085 0,1275 0,0425 ( mol )
\(m_{Al_2S_3}=0,0425.150=6,375g\)
\(m_{Al\left(dư\right)}=\left(0,11-0,085\right).27=0,675g\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{Al_2S_3}=\dfrac{6,375}{6,375+0,675}.100=90,42\%\\\%m_{Al}=100\%-90,42\%=9,58\%\end{matrix}\right.\)
Check lại giúp mình câu b với:((