bài 13
nP2O5=28,4/142=0,2 mol
P2O5 + 3H2O --> 2H3PO4
0,2 0,4 mol
=>C M =0,4/0,5=0.8 M
4Mg +6H3PO4 --> 2Mg2(PO4)3 + 9H2
4/15 0,4 mol
=> mMg=4/15*24=6,4 g
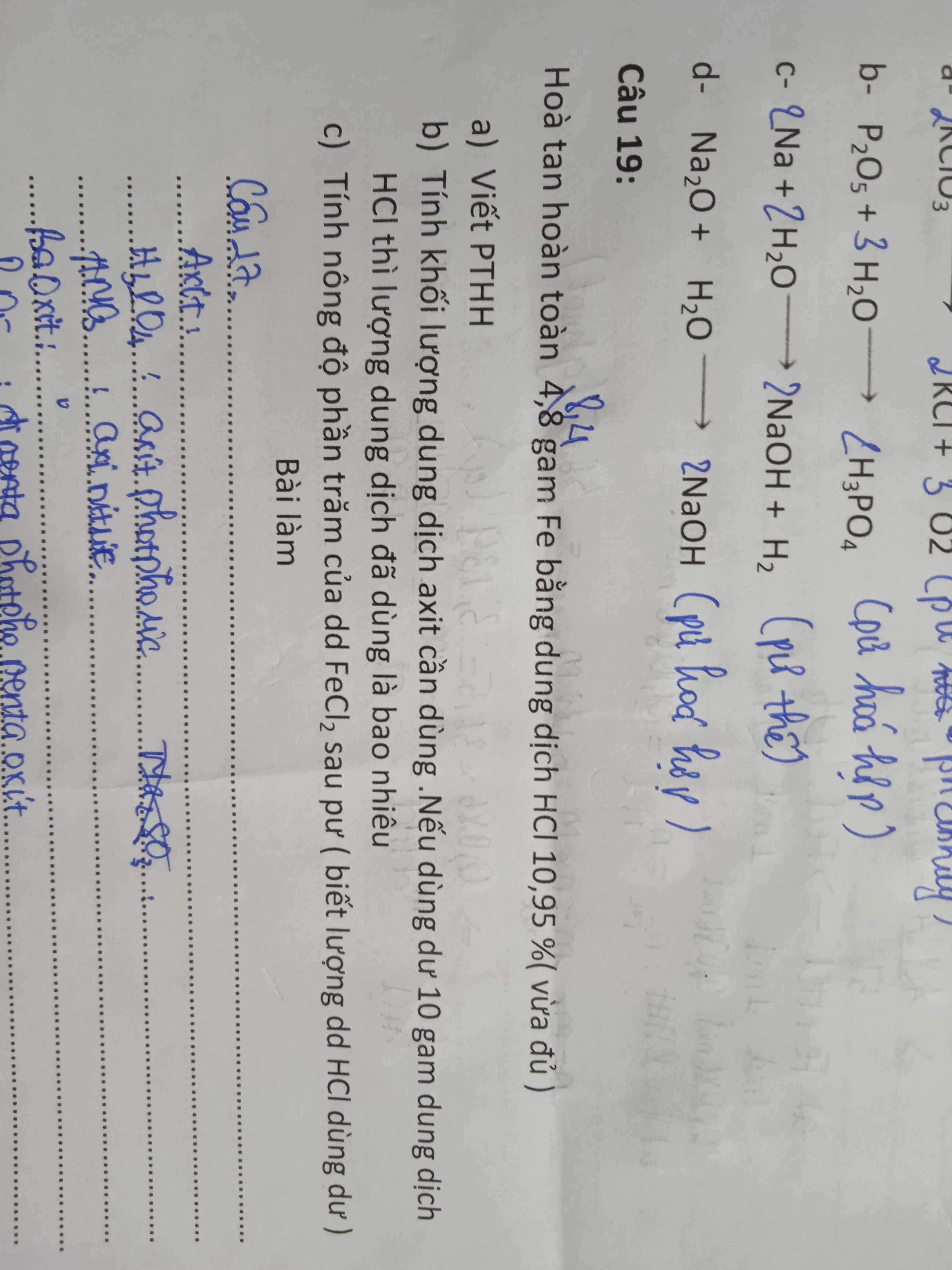
Bài 13:
a) PTHH: \(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
Theo PTHH: \(n_{H_3PO_4}=2n_{P_2O_5}=2\cdot\dfrac{28,4}{142}=0,4\left(mol\right)\)
\(\Rightarrow C_{M_{H_3PO_4}}=\dfrac{0,4}{0,5}=0,8\left(M\right)\)
b) PTHH: \(3Mg+2H_3PO_4\rightarrow Mg_3\left(PO_4\right)_2+3H_2\uparrow\)
Theo PTHH: \(n_{Mg}=\dfrac{3}{2}n_{H_3PO_4}=1,2\left(mol\right)\)
\(\Rightarrow m_{Mg}=1,2\cdot24=28,8\left(g\right)\)
Bài 14:
a+b) PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
Ta có: \(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)=n_{H_2}\)
\(\Rightarrow\left\{{}\begin{matrix}n_{HCl}=0,6\left(mol\right)\\n_{H_2}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C\%_{HCl}=\dfrac{0,6\cdot36,5}{400}\cdot100\%=5,475\%\\V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\end{matrix}\right.\)
c) PTHH: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CuO}=\dfrac{16}{80}=0,2\left(mol\right)\\n_{H_2}=0,3\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\) Hidro dư 0,1 mol
\(\Rightarrow m_{H_2}=0,1\cdot2=0,2\left(g\right)\)
Bài 15:
PTHH: \(NaOH+HCl\rightarrow NaCl+H_2O\)
a) Ta có: \(n_{NaOH}=\dfrac{200\cdot20\%}{40}=1\left(mol\right)=n_{NaCl}\)
\(\Rightarrow C\%_{NaCl}=\dfrac{1\cdot58,5}{200+100}\cdot100\%=19,5\%\)
b) Theo PTHH: \(n_{HCl}=n_{NaOH}=1\left(mol\right)\)
\(\Rightarrow C\%_{HCl}=\dfrac{1\cdot36,5}{100}\cdot100\%=36,5\%\)