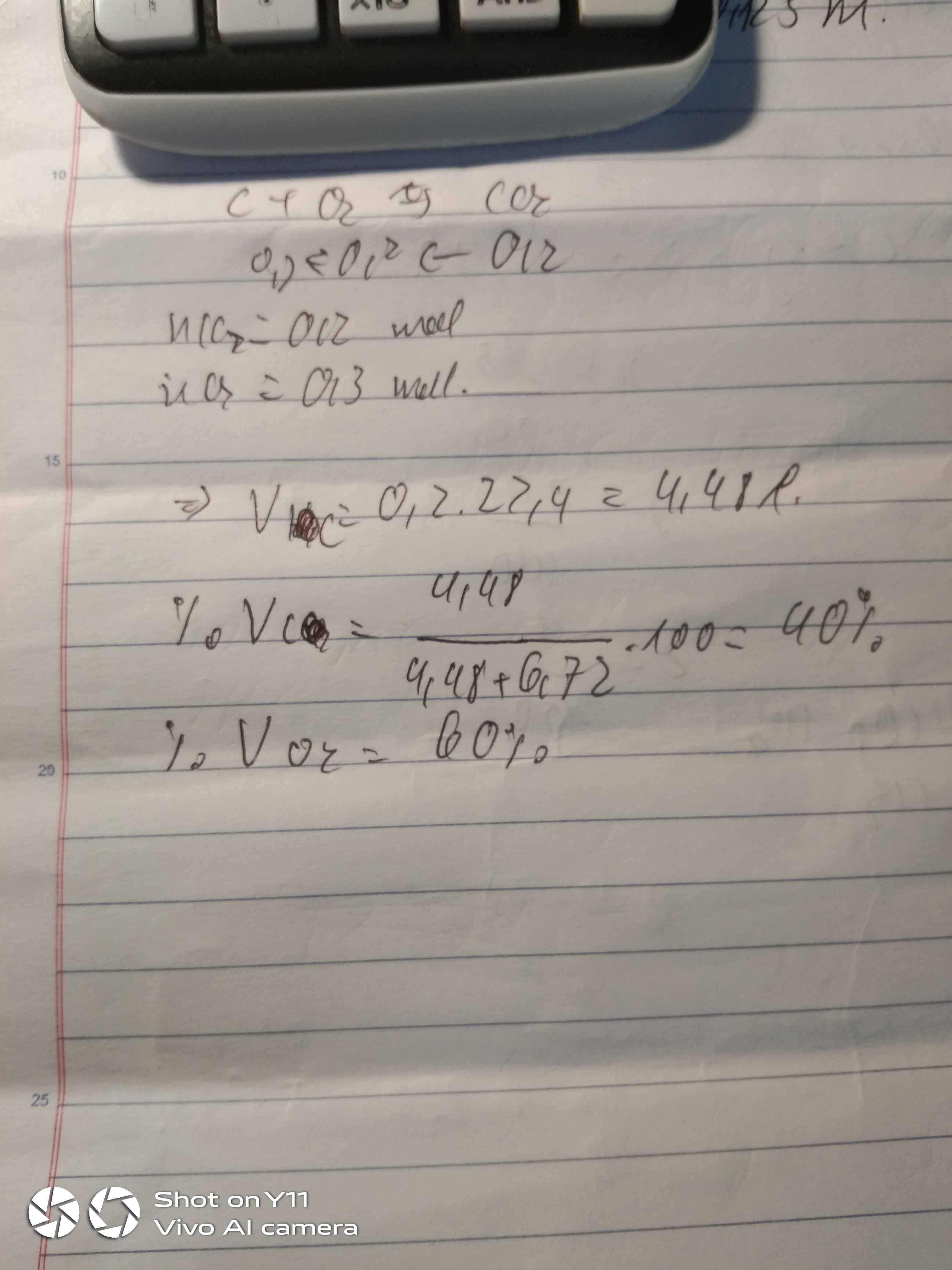\(n_{H_2O}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
\(2H_2+O_2\underrightarrow{t^o}2H_2O\)
0,2 0,2
=> \(V_{H_2}=0,2.22,4=4,48\left(l\right)\\
\%V_{H_2}=\dfrac{4,48}{4,48+6,72}.100\%=40\%\\
\Rightarrow\%V_{O_2}=100\%-40\%=60\%\)
Đúng 0
Bình luận (1)
Các câu hỏi tương tự
Đốt cháy hoàn toàn một hỗn hợp khí Z gồm CO và H 2 cần dùng 4,48 lít khí O 2 (đktc). Thể tích khí sinh ra chứa 3,36 lít C O 2 . Hãy tính thành phần phần trăm theo thể tích mỗi khí trong hỗn hợp ban đầu.
đôt cháy hoàn toàn hỗn hợp khí gồm co và h2 cần dùng 6,72 lít khí o2.thu được 4,48 lít co2.tính phần trăm theo khối lượng và thể tích của hỗn hợp ban đầu
Đốt cháy hoàn toàn 6,72 lít hỗn khí gas gồm CH4 và C4H10 trong không khí thu được CO2 và hơi nước.Biết trong hỗn hợp có V-CH4 : V-C4H10=1:2
a)Viết PTHH
b)Tính: V không khí cần dùng và V-CO2 sinh ra.Thể tích các khí được đo ở điều kiện tiêu chuẩn
Mọi người giải hộ em chứ hôm sau nữa em phải nộp rồi
đốt cháy hoàn toàn 13g hỗn hợp gồm C2H4, C3H6, C2H4O2 thì cần V lít khí O2 sau phản ứng thu được hỗn hợp gồm 15,68l khí co2 và h2o(đktc)
a, viết PTHH
b, Tính V
c, Tính % khối lượn C2H4O2
Đốt cháy hoàn toàn một hỗn hợp khí gồm có CO và H2 cần dùng 7,437 lít khí O2 khí sinh ra có 4,958 lít khí CO2. Thành phần phần trăm theo thể tích của khí H2 trong hỗn hợp bạn đầu là
Đốt cháy hoàn toàn hỗn hợp A gồm CO và H2 bằng một lượng vừa đủ. Sau khi phản ứng xảy ra hoàn toàn, ngưng tụ sản phẩm thu được 12,6 gam H2O và 13,44 lít khí CO2(đktc)
a. PTHH
b. Tính thể tích mỗi khí trong hỗn hợp A và thể tích O2 đã dùng
c. Tính tỉ khối của hỗn hợp A so với O2
Đốt cháy hoàn toàn 3,36 lit hỗn hợp C2H4, C3H6 trong bình chứa O2 dư. Sau khi phản ứng kết thúc người ta thu được 17,6 gam khí CO2 và hơi nước
a. Tính %V mỗi khí trong hỗn hợp ban đầu
b.Tính thể tích khí O2 đã tham gia phản ứng. Các khí đo ở đktc
Đốt cháy hoàn toàn 14,8 g hỗn hợp khí gồm CO và H2 cần dùng 10,08 l khí O2 Tính:
a. Thành phần % về thể tích mỗi khí trong hỗn hợp ban đầu(đktc)
b. Thể tích khí CO2 và khối lượng nước thu được(đktc)
đốt cháy hoàn toàn m gam Cacbon trong bình chứa V lít khí oxi (đktc) sau phản ứng thu được hỗn hợp khí A có tỷ khối đối với O2 là 1,25. a) hãy xác định thành phần phần trăm theo thể tích các khí có trong hỗn hợp A? b) Tính m và V. Biết rằng khi dẫn hỗn hợp A vào bình đựng dung dịch Ca(OH)2 dư thì thu được 6 gam CaCO3 kết tủa trắng?