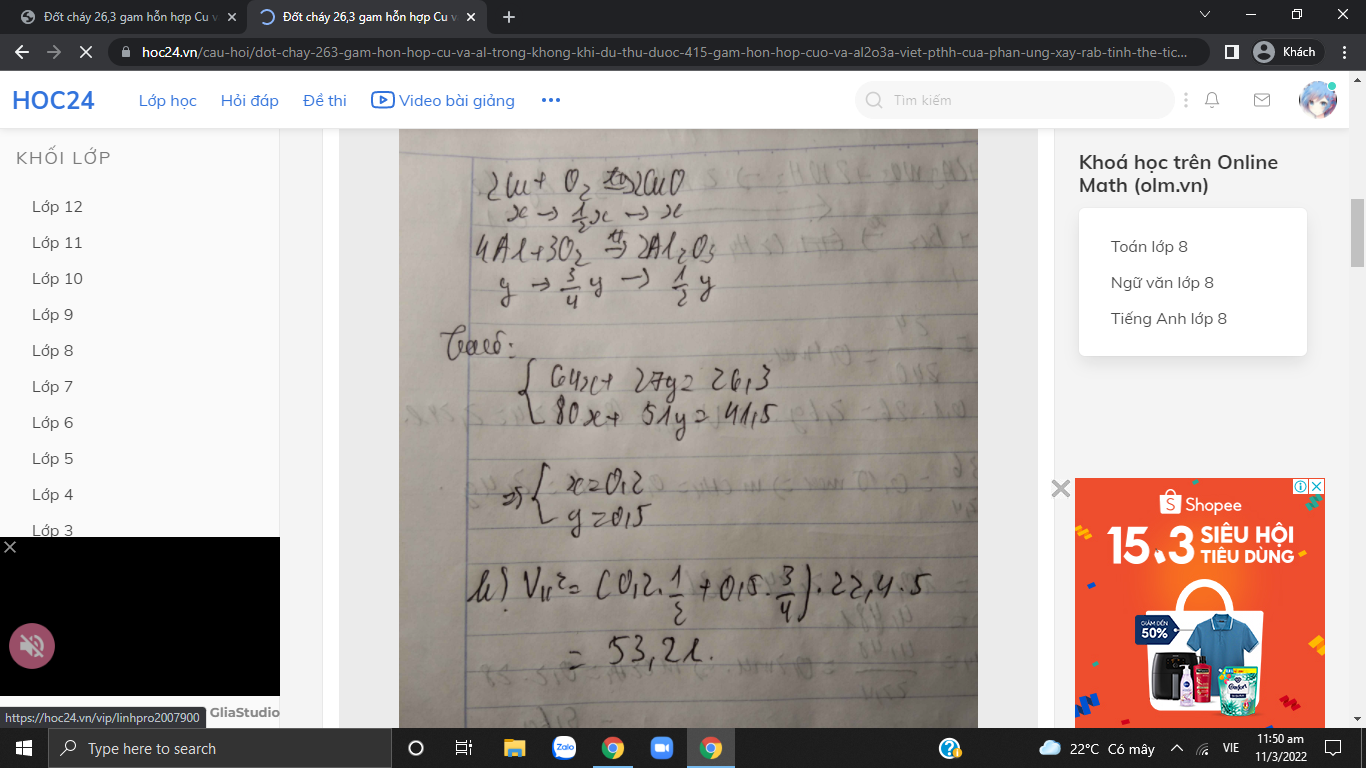
a.
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\) \(4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\)
x 1/2x x y 3/4y 1/2y ( mol )
Gọi \(\left\{{}\begin{matrix}n_{Cu}=x\\n_{Al}=y\end{matrix}\right.\)
Ta có:
\(\left\{{}\begin{matrix}64x+27y=26,3\\80x+\dfrac{1}{2}y.102=41,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,5\end{matrix}\right.\)
\(V_{kk}=\left(\dfrac{1}{2}.0,2+\dfrac{3}{4}.0,5\right).22,4.5=0,475.112=53,2l\)