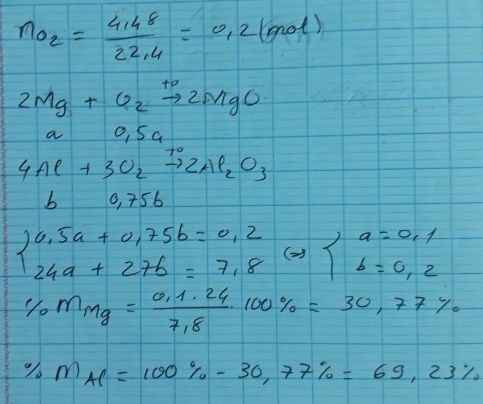\(n_{O_2}=0,2\left(mol\right)\)
PTHH:
\(2Mg+O_2\rightarrow2MgO\)
\(4Al+3O_2\rightarrow2Al_2O_3\)
\(\Rightarrow n_{O_2}=\dfrac{1}{2}n_{Mg}+\dfrac{3}{4}n_{Al}\Leftrightarrow\dfrac{1}{2}n_{Mg}+\dfrac{3}{4}n_{Al}=0,2\left(1\right)\)
Mặt khác: \(24n_{Mg}+27n_{Al}=7,8\left(2\right)\)
Giải hệ hai phương trình (1) và (2) ta được:
\(\left\{{}\begin{matrix}n_{Mg}=0,1\left(mol\right)\\n_{Al}=0,2\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,1.24}{7,8}.100\%=30,77\%\\\%m_{Al}=\dfrac{0,2.27}{7,8}.100\%=69,23\%\end{matrix}\right.\)