\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(\Rightarrow n_{MOH}=2\cdot0.15=0.3\left(mol\right)\)
\(n_{MOH\left(cd\right)}=\dfrac{0.3}{2}=0.15\left(mol\right)\)
\(MOH+HCl\rightarrow MCl+H_2O\)
\(0.15.........0.15\)
\(V_{dd_{HCl}}=\dfrac{0.15}{2}=0.075\left(l\right)\)







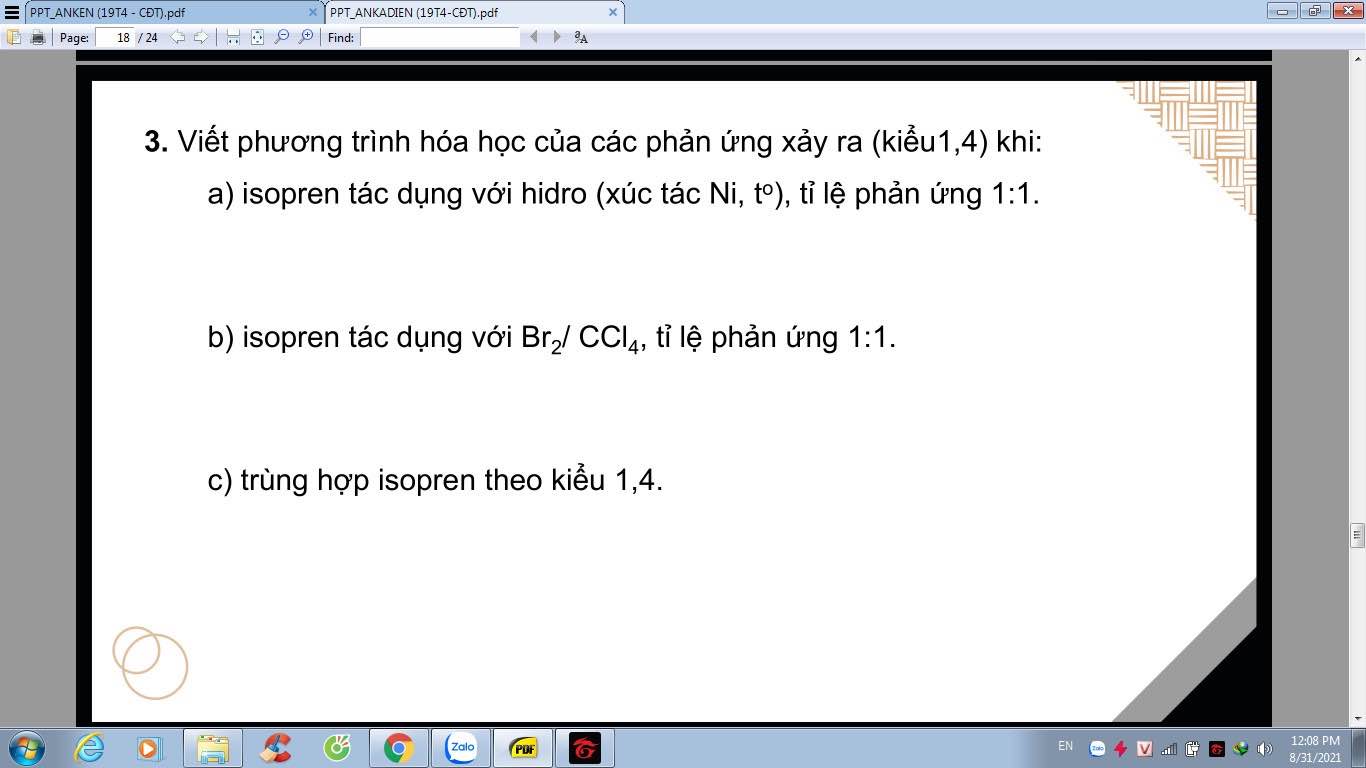




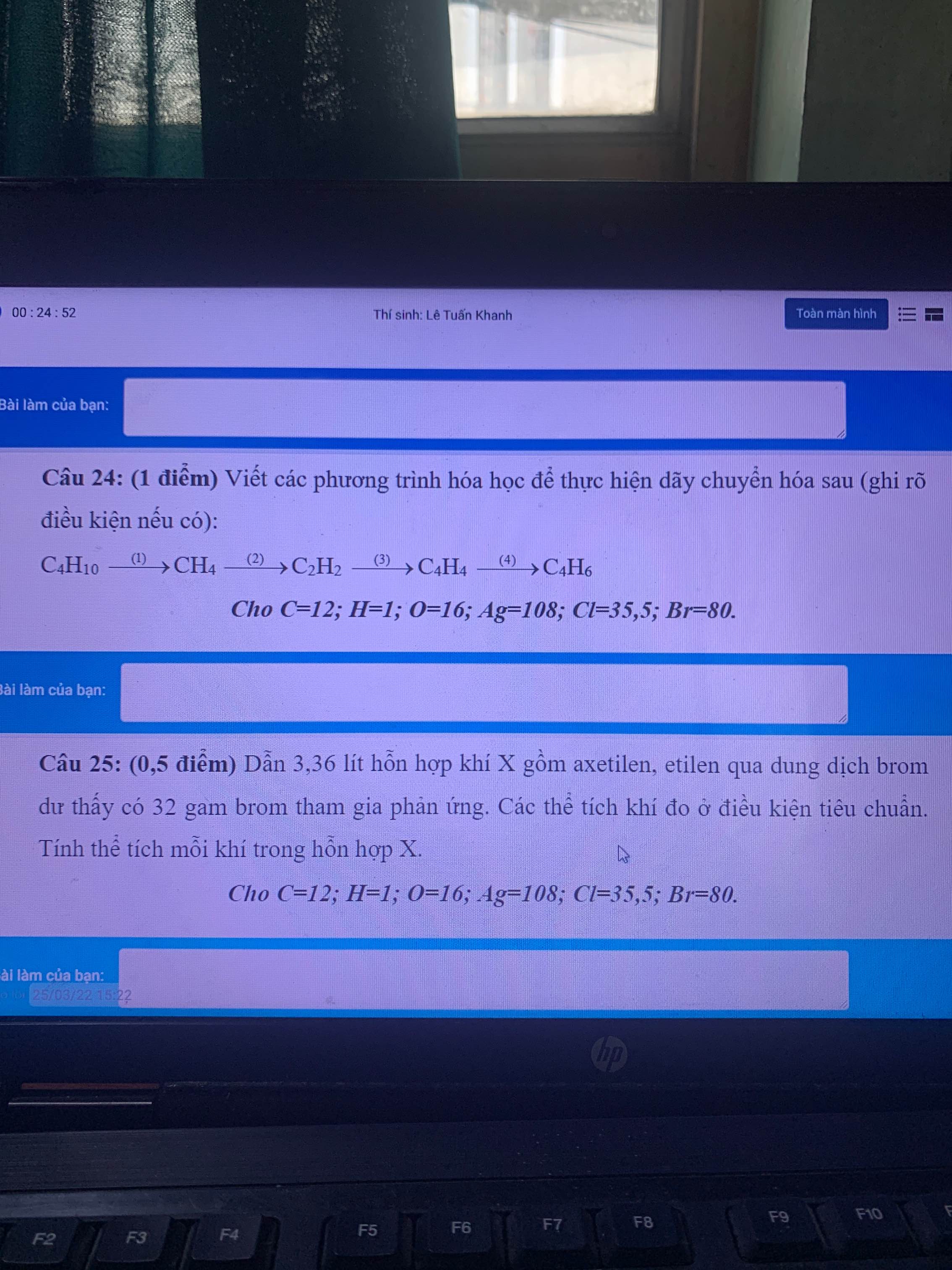




 giúp em gọi tên thây thế của các ankan câu c e g với ạ
giúp em gọi tên thây thế của các ankan câu c e g với ạ