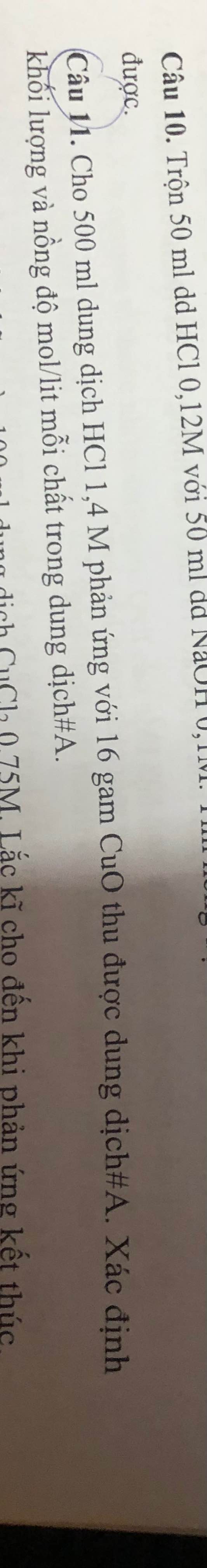\(n_{HCl}=1,4.0,5=0,7\left(mol\right)\\ n_{CuO}=\dfrac{16}{80}=0,2\left(mol\right)\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ Vì:\dfrac{0,7}{2}>\dfrac{0,2}{1}\Rightarrow HCldư\\ \Rightarrow ddsau:\left\{{}\begin{matrix}HCl\left(dư\right)\\CuCl_2\end{matrix}\right.\\ V_{ddsau}=V_{ddHCl}=500\left(ml\right)=0,5\left(l\right)\\ n_{CuCl_2}=n_{CuO}=0,2\left(mol\right)\\ n_{HCl\left(dư\right)}=0,7-0,2.2=0,3\left(mol\right)\\ m_{CuCl_2}=135.0,2=27\left(g\right)\\ m_{HCl\left(dư\right)}=0,3.36,5=10,95\left(g\right)\\ C_{MddHCl}=\dfrac{0,3}{0,5}=0,6\left(M\right)\\ C_{MddCuCl_2}=\dfrac{0,2}{0,5}=0,4\left(M\right)\)
Đúng 3
Bình luận (0)