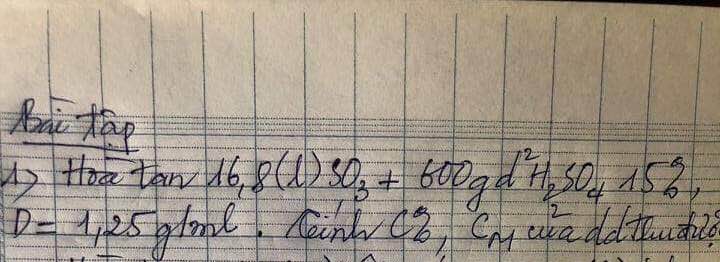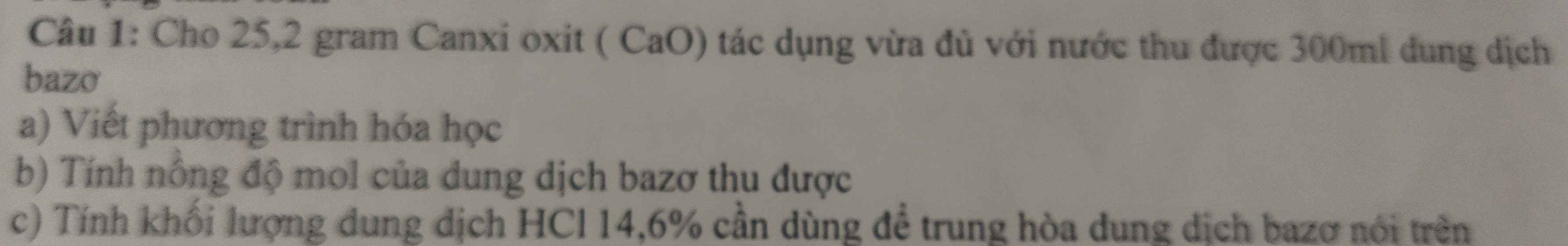Bài 3:
a: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
a a
\(4Al+3H_2SO_4\rightarrow2Al_2\left(SO_4\right)_3+3H_2\)
b 3/2b
Theo đề, ta có \(\left\{{}\begin{matrix}65a+27b=3.79\\a+\dfrac{3}{2}b=0.08\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0.05\\b=0.02\end{matrix}\right.\)
\(m_{Zn}=0.05\cdot65=3.25\left(g\right)\)
\(m_{Al}=0.02\cdot27=0.54\left(g\right)\)
b: \(\%m_{Zn}=\dfrac{3.25}{3.79}\simeq85.75\%\)
=>%mAl=14,25%