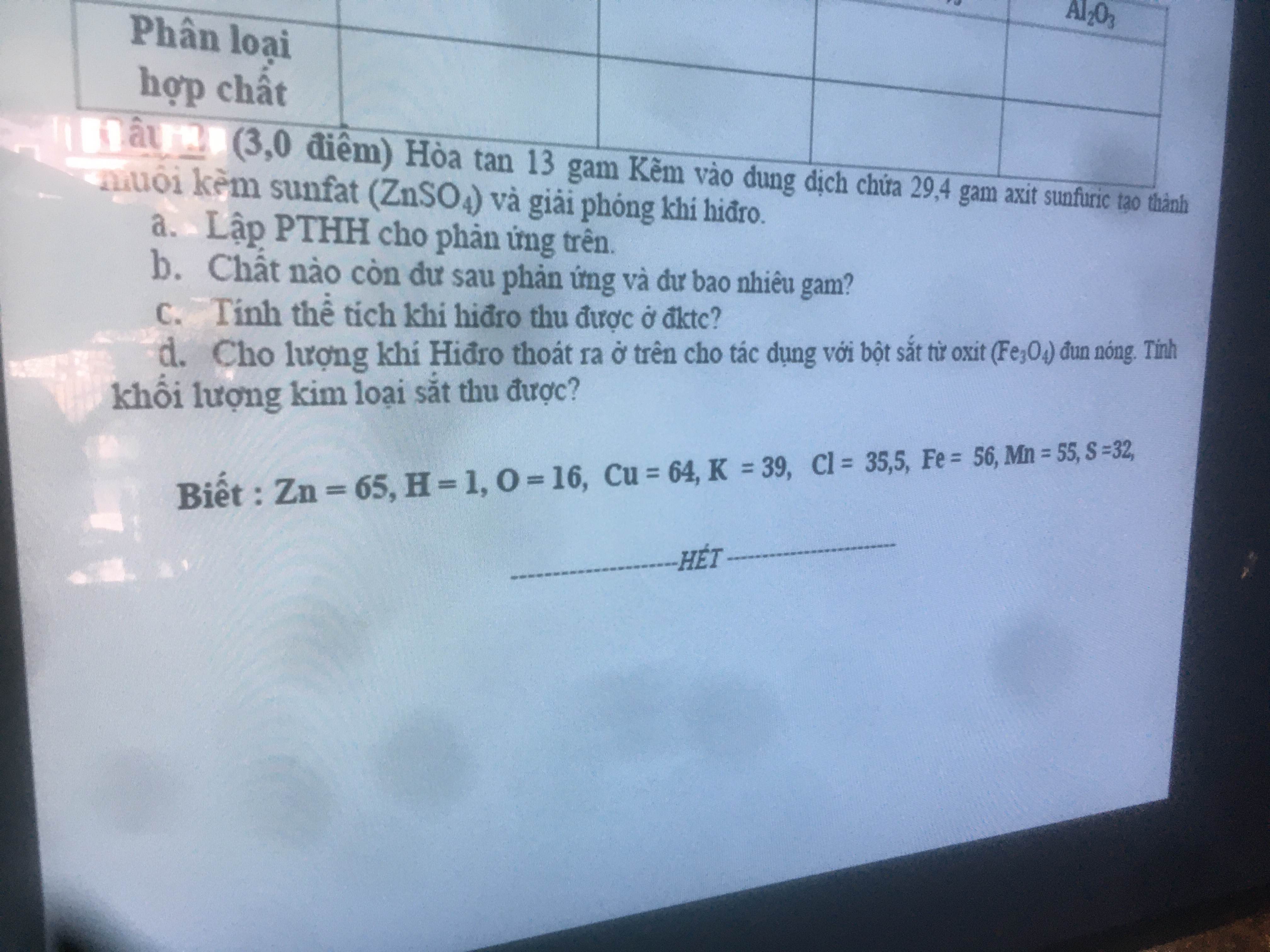
\(n_{Zn}=\dfrac{13}{65}=0.2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{29.4}{98}=0.3\left(mol\right)\)
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(1..........1\)
\(0.2...........0.3\)
\(LTL:\dfrac{0.2}{1}< \dfrac{0.3}{1}\Rightarrow H_2SO_4dư\)
\(m_{H_2SO_4\left(dư\right)}=\left(0.3-0.2\right)\cdot98=9.8\left(g\right)\)
\(V_{H_2}=0.2\cdot22.4=4.48\left(l\right)\)
\(Fe_3O_4+4H_2\underrightarrow{^{t^0}}3Fe+4H_2O\)
\(.........0.2.....0.15\)
\(m_{Fe}=0.15\cdot56=8.4\left(g\right)\)