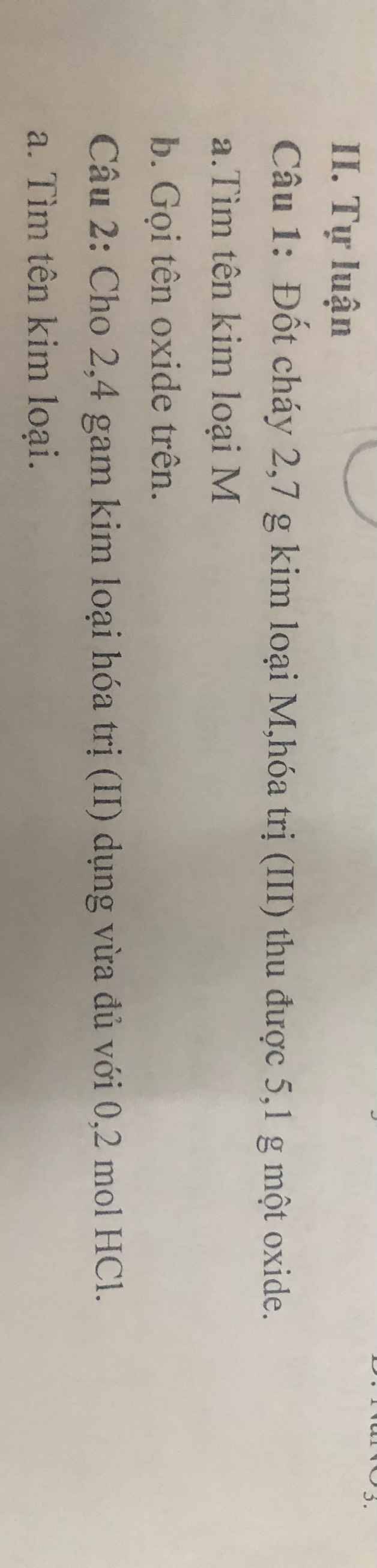\(1.a.CTHH:M_2O_3\\ 4M+3O_2\xrightarrow[]{t^0}2M_2O_3\\ \Rightarrow n_M:4=n_{M_2O_3}:2\\ \Rightarrow\dfrac{2,7}{M}:4=\dfrac{5,1}{2M+48}:2\\ \Rightarrow M=27,Al\\ \Rightarrow CTHH:Al_2O_3\\ bAl_2O_3:Alumium.oxide\\ 2.KL:R\\ R+2HCl\rightarrow RCl_2+H_2\\ n_R=\dfrac{1}{2}n_{HCl}=0,1mol\\ M_R=\dfrac{2,4}{0,1}=24\\ M.là.Mg\)
Đúng 1
Bình luận (0)
Các câu hỏi tương tự
cho CTHH: CxHy +\(\left(x+\dfrac{y}{4}\right)\) O2 --------> xCO2 + \(\dfrac{y}{2}\) H2O
Biết tổng số mol CO2 và H2O là 5 vầ tích số mol của CxHy và O2 là 0,35
Tìm CxHy
Tổng số hạt mang điện trong phân tử XY2 là 64. Sốhatj không mang điện trong hạt nhân nguyên tủ X nhiều hơn số hạt mang điện tronghatj nhân nguyên tử Y là 8
A. Tìm nguyên tố X,Y
B. Viết cthh và đọc tên XY2
Nung nóng 36,75g kaliclorat thu được khí A . Đốt cháy dây sắt trong bình chứa toàn bộ A sinh ra ở trên thu được chất rắn B a) viết PTHH sảy ra- b) tính thể tích khí A sinh ra(đktc) - c) tính khối lượng chất rắn B thu được
Hợp chất nào sau đây không phải là oxit
A. C O 2
B. S O 2
C. CuO
D. CuS
Bazo tương ứng của MgO A.
M
g
O
H
2
B.
M
g
C
l
2
C.
M
g
S
O
4
D.
M
g
O
H
3
Đọc tiếp
Bazo tương ứng của MgO
A. M g O H 2
B. M g C l 2
C. M g S O 4
D. M g O H 3
Chọn đáp án đúng nhất. Bản chất của phản ứng cháy là:
A. Là phản ứng hóa hợp.
B. Sản phẩm tạo ra có C O 2
C. Là phản ứng oxi hóa – khử
D. Là phản ứng thu nhiệt
Khí nào nặng nhất trong các khí sau
A. C H 4
B. C O 2
C. N 2
D. H 2
Số mol C, O 2 , Fe tương ứng của 4,8 gam C; 16 gam O 2 ; 0,56 gam Fe là
A. 0,04 mol; 0,5 mol; 0,1 mol
B. 0,4 mol; 0,5 mol; 0,01 mol
C. 4 mol; 5 mol; 1 mol
D. 0,4 mol; 0,1 mol; 0,3 mol
Các chất dùng để điều chế Oxi trong phòng thí nghiệm là
A. K C l O 3
B. K M n O 4
C. C a C O 3
D. Cả A & B
Chọn đáp án sai
A. Có 3 bước lập phương trình hóa học
B. Phương trình hóa học biểu diễn ngắn gọn phản ứng hóa học
C. Muối ăn có công thức hóa học là NaCl
D.Ý nghĩa của phương trình hóa học là cho biết nguyên tố nguyên tử