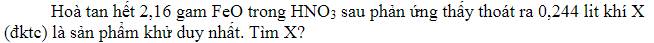\(n_{FeO}=\dfrac{2,16}{72}=0,03mol\\ n_X=\dfrac{0,224}{22,4}=0,01mol\\ \overset{+2}{Fe}O\underrightarrow{\overset{+5}{+HNO_3}}\overset{+3}{Fe}\left(NO_3\right)_3+\overset{z}{X}+H_2O\\ BTe:n_{FeO}=\left(5-Z\right)n_X\\ \Leftrightarrow0,03=\left(5-Z\right)0,01\\ \Leftrightarrow Z=+2\\ \Rightarrow X=NO\)
Đúng 2
Bình luận (4)
Các câu hỏi tương tự
tính thành phần phần trăm các nguyên tố trong hợp chất C2H4
Cho 2 chất hữu cơ A và B có công thức phân tử lần lượt là C3H8O và C3H6O2. Biết rằng chất A và chất B đều tác dụng với Na, chỉ có chất B tác dụng với NaHCO3. a. Xác định các công thức cấu tạo có thể có của A và B b. Viết các phương trìn hóa học xảy ra khi cho A tác dụng với B.
Đọc tiếp
Cho 2 chất hữu cơ A và B có công thức phân tử lần lượt là C3H8O và C3H6O2. Biết rằng chất A và chất B đều tác dụng với Na, chỉ có chất B tác dụng với NaHCO3.
a. Xác định các công thức cấu tạo có thể có của A và B
b. Viết các phương trìn hóa học xảy ra khi cho A tác dụng với B.
Để đốt cháy V lí khí metan thu được 7.2 gam hơi nước A tính V lít khí metan B bao nhiêu lít kk cần dùng , biết rằng õit chiếm 20 phần trăm thể tích kk . Thể tích các khí đó ở đktc C dẫn toàn bộ khí thu được ở trên qua dd Barihiđroxit dư , sau pứ thu được bao nhiêu gam kết tủa ?
Cho 500ml dung dịch axit axetic tác dụng hoàn toàn với Mg, thu được 14,2g muối (CH3COO)2Mg
a/ Tính nồng độ mol của dung dịch axit
b/ Tính thể tích khí hidro sinh ra ở đktc
c/ Tính thể tích dung dịch NaOH 0,5M cần dùng để trung hòa 500ml dung dịch axit trên
Đốt cháy 0,9g chất hữu cơ A thu được co2 và h2o và khí nitơ.Cho sản phẩm qua dd ca(oh)2 khối lượng bình tăng 3,02g xuất hiện 4g kết tủa và 0,224l khí thoát ra ở đktc. Xác định công thức A
Trung hoà 20 ml dung dịch H 2 SO 4 1M bằng dung dịch NaOH 20%. Nếu trung hoà dung dịch axit sunfuric trên bằng dung dịch KOH 5,6%, có khối lượng riêng là 1,045 g/ml, thì cần bao nhiêu ml dung dịch KOH ?
Hoà tan hoàn toàn 0,56 gam sắt bằng dung dịch H 2 SO 4 loãng 19,6% vừa đủ. Viết phương trình hoá học
Cho hỗn hợp X gồm 3 kim loại K, Al và Fe vào nước dư thu được dung dịch Y, phần không tan Z có khối lượng 11,15 gam và 6,72 lít H2 (đktc). Cho Z vào 100 ml dung dịch CuSO4 3M thu được 16 gam chất rắn T. Xác định khối lượng của mỗi kim loại trong X, biết rằng các phản ứng đều xảy ra hoàn toàn.
Hỗn hợp X gồm ba kim loại Al, Fe, Cu. Cho m gam hỗn hợp X vào dung dịch CuSO4 (dư) sau khi phản ứng xảy ra hoàn toàn thu được 35,2 gam kim loại. Nếu cũng hòa tan m gam hỗn hợp X vào 500 ml dung dịch HCl 2M đến khi phản ứng xảy ra hoàn toàn thu được 8,96 lít khí H2 (đktc), dung dịch Y và a gam chất rắn.Cho từ từ dung dịch NaOH 2M vào dung dịch Y và khuấy đều đến khi thấy kết tủa bắt đầu xuất hiện thi dùng hết V1 lít dung dịch NaOH 2M, tiếp tục cho dung dịch NaOH 2M trên vào đến khi lượng kết tủa...
Đọc tiếp
Hỗn hợp X gồm ba kim loại Al, Fe, Cu. Cho m gam hỗn hợp X vào dung dịch CuSO4 (dư) sau khi phản ứng xảy ra hoàn toàn thu được 35,2 gam kim loại. Nếu cũng hòa tan m gam hỗn hợp X vào 500 ml dung dịch HCl 2M đến khi phản ứng xảy ra hoàn toàn thu được 8,96 lít khí H2 (đktc), dung dịch Y và a gam chất rắn.Cho từ từ dung dịch NaOH 2M vào dung dịch Y và khuấy đều đến khi thấy kết tủa bắt đầu xuất hiện thi dùng hết V1 lít dung dịch NaOH 2M, tiếp tục cho dung dịch NaOH 2M trên vào đến khi lượng kết tủa không có sự thay đổi nữa thì thể tích dung dịch NaOH đã dùng hết là 600 ml. Tìm các giá trị m và V1
Hỗn hợp A gồm CaCO3, Cu, FeO và Al. Nung nóng A (trong điều kiện không có không khí) một thời gian thu được chất rắn B. Cho B vào nước dư, thu được dung dịch C và chất rắn D (không thay đổi khối lượng khi cho vào dung dịch NaOH). Cho D tác dụng với dung dịch H2SO4 đặc nóng, dư. Xác định thành phần của B, C, D và viết các phương trình phản ứng xảy ra.
Đọc tiếp
Hỗn hợp A gồm CaCO3, Cu, FeO và Al. Nung nóng A (trong điều kiện không có không khí) một thời gian thu được chất rắn B. Cho B vào nước dư, thu được dung dịch C và chất rắn D (không thay đổi khối lượng khi cho vào dung dịch NaOH). Cho D tác dụng với dung dịch H2SO4 đặc nóng, dư. Xác định thành phần của B, C, D và viết các phương trình phản ứng xảy ra.