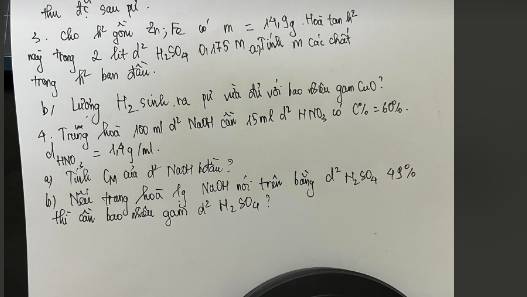Bài 3:
a, \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
Gọi: \(\left\{{}\begin{matrix}n_{Zn}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\) ⇒ 65x + 56y = 14,9 (g) (1)
Theo PT: \(n_{H_2SO_4}=n_{Zn}+n_{Fe}=x+y=2.0,175=0,35\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=-\dfrac{47}{90}\\y=\dfrac{157}{180}\end{matrix}\right.\)
Đến đây thì ra số mol âm, bạn xem lại đề nhé.
Bài 4:
a, Ta có: \(m_{ddHNO_3}=1,4.15=21\left(g\right)\Rightarrow m_{HNO_3}=21.60\%=12,6\left(g\right)\)
\(\Rightarrow n_{HNO_3}=\dfrac{12,6}{63}=0,2\left(mol\right)\)
PT: \(NaOH+HNO_3\rightarrow NaNO_3+H_2O\)
Theo PT: \(n_{NaOH}=n_{HNO_3}=0,2\left(mol\right)\)
\(\Rightarrow C_{M_{NaOH}}=\dfrac{0,2}{0,1}=2\left(M\right)\)
b, PT: \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
Theo PT: \(n_{H_2SO_4}=\dfrac{1}{2}n_{NaOH}=0,1\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,1.98=9,8\left(g\right)\Rightarrow m_{ddH_2SO_4}=\dfrac{9,8}{49\%}=20\left(g\right)\)