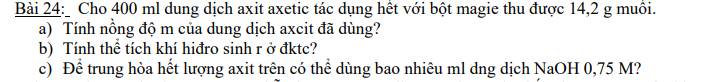a) $n_{(CH_3COO)_2Mg} = \dfrac{14,2}{142} = 0,1(mol)$
$2CH_3COOH + Mg \to (CH_3COO)_2Mg + H_2$
Theo PTHH : $n_{CH_3COOH} = 2n_{(CH_3COO)_2Mg} = 0,2(mol)$
$C_{M_{CH_3COOH}} = \dfrac{0,2}{0,4} = 0,5M$
b) $n_{H_2} = n_{(CH_3COO)_2Mg} = 0,1(mol)$
$V_{H_2} = 0,1.22,4 = 2,24(lít)$
c)
$CH_3COOH + NaOH \to CH_3COONa + H_2O$
$n_{NaOH} = n_{CH_3COOH} = 0,1(mol)$
$V_{dd\ NaOH} = \dfrac{0,1}{0,75} = 0,13(lít)$