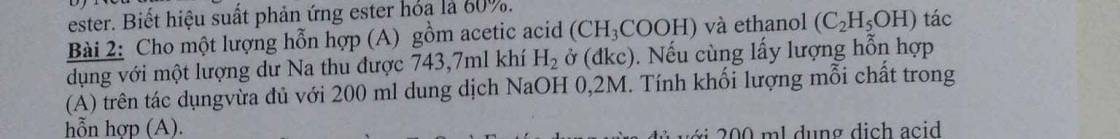$CH_3COOH + NaOH \to CH_3COONa + H_2O$
$n_{CH_3COOH} = n_{NaOH} = 0,2.0,2 = 0,04(mol)$
$\Rightarrow m_{CH_3COOH} = 0,04.60 = 2,4(gam)$
$n_{H_2} = \dfrac{743,7}{1000.24,79} =0,03(mol)$
$2CH_3COOH + 2Na \to 2CH_3COONa + H_2$
$2C_2H_5OH + 2Na \to 2C_2H_5ONa + H_2$
$n_{H_2} = \dfrac{1}{2}n_{CH_3COOH} + \dfrac{1}{2}n_{C_2H_5OH}$
$\Rightarrow n_{C_2H_5OH} = 0,02(mol)$
$\Rightarrow m_{C_2H_5OH} = 0,02.46 = 0,92(gam)$