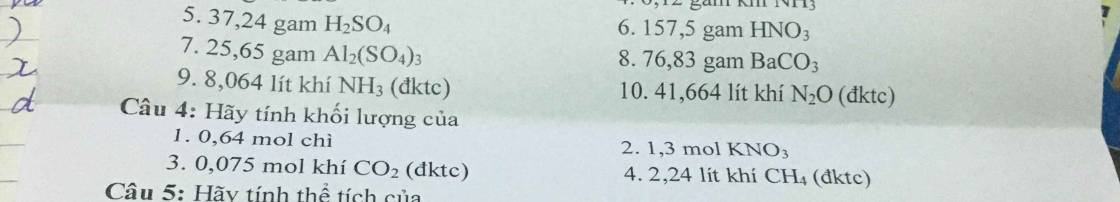Câu 4:
1) \(m_{Pb}=n.M=0,64.207=132,48\left(g\right)\)
2) \(m_{KNO_3}=n.M=1,3.101=131,3\left(g\right)\)
3) \(m_{CO_2}=n.M=0,075.44=3,3\left(g\right)\)
4) \(n_{CH_4}=\dfrac{V_{\left(đktc\right)}}{22,4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
=> \(m_{CH_4}=n.M=0,1.16=1,6\left(g\right)\)