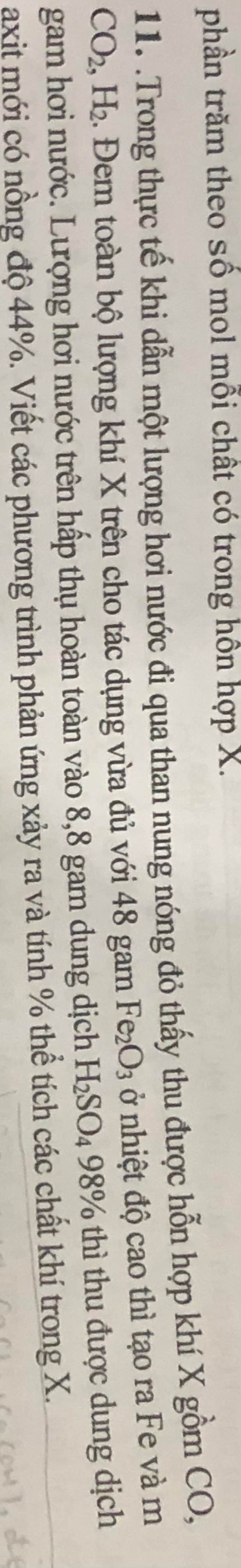PTHH:
\(H_2O+C\xrightarrow[]{t^o}CO+H_2\) (1)
\(2H_2O+C\xrightarrow[]{t^o}CO_2+2H_2\) (2)
\(3H_2+Fe_2O_3\xrightarrow[]{t^o}2Fe+3H_2O\) (3)
\(3CO+Fe_2O_3\xrightarrow[]{t^o}2Fe+3CO_2\) (4)
Ta có: \(\left\{{}\begin{matrix}n_{Fe_2O_3}=\dfrac{48}{160}=0,3\left(mol\right)\\n_{H_2SO_4}=\dfrac{8,8.98\%}{98}=0,088\left(mol\right)\end{matrix}\right.\)
=> \(m_{ddH_2SO_4\left(mới\right)}=\dfrac{0,088.98}{44\%}=19,6\left(g\right)\)
=> \(m_{H_2O}=19,6-8,8=10,8\left(g\right)\Rightarrow n_{H_2O}=\dfrac{10,8}{18}=0,6\left(mol\right)\)
Theo PT (3), (4): \(n_{H_2}=n_{H_2O}=0,6\left(mol\right)\)
\(3n_{Fe_2O_3}=n_{CO}+n_{H_2O}\Rightarrow n_{CO}=3.0,3-0,6=0,3\left(mol\right)\)
Theo PT (1), (2): \(n_{H_2}=n_{CO}+2n_{CO_2}\)
`=>` \(n_{CO_2}=\dfrac{0,6-0,3}{2}=0,15\left(mol\right)\)
`=>` \(\left\{{}\begin{matrix}\%V_{CO}=\dfrac{0,3}{0,3+0,6+0,15}.100\%=28,57\%\\\%V_{CO_2}=\dfrac{0,15}{0,3+0,6+0,15}.100\%=14,29\%\\\%V_{H_2}=\left(100-28,57-14,29\right)\%=57,14\%\end{matrix}\right.\)