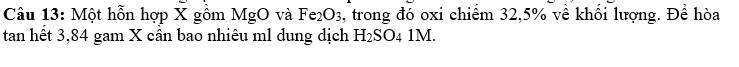Gọi \(x,y\) lần lượt là số mol của \(MgO,Fe_2O_3\)
\(\Rightarrow40x+160y=3,84\Leftrightarrow x+4y=0,096\left(1\right)\)
Oxi chiếm 32,5% \(m_{hh}\)\(\Rightarrow16x+16.3y=32,5\%.3,84\)
\(\Leftrightarrow16x+48y=1,248\)
\(\Leftrightarrow x+3y=0,078\left(2\right)\)
\(\left(1\right)\left(2\right)\Rightarrow\left\{{}\begin{matrix}x=0,024\left(mol\right)\\y=0,018\left(mol\right)\end{matrix}\right.\)
\(MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
0,024 0,024 (mol)
\(Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
0,018 0,054 (mol)
\(n_{H_2SO_4}=0,024+0,054=0,078\left(mol\right)\)
\(V_{ddH_2SO_4}=\dfrac{0,078}{1}=0,078\left(l\right)=78\left(ml\right)\)