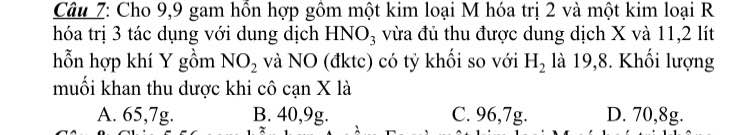\(\overline{M_Y}=19,8.2=39,6\left(g/mol\right)\)
Áp dụng sơ đồ đường chéo, ta có: \(\dfrac{n_{NO_2}}{n_{NO}}=\dfrac{39,6-30}{46-39,6}=\dfrac{3}{2}\)
Mà \(n_{NO_2}+n_{NO}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
=> \(\left\{{}\begin{matrix}n_{NO}=\dfrac{2}{2+3}.0,5=0,2\left(mol\right)\\n_{NO_2}=0,5-0,2=0,3\left(mol\right)\end{matrix}\right.\)
PTHH:
\(M+4HNO_3\rightarrow M\left(NO_3\right)_2+2NO_2+2H_2O\) (1)
\(3M+8HNO_3\rightarrow3M\left(NO_3\right)_2+2NO+4H_2O\) (2)
\(R+6HNO_3\rightarrow R\left(NO_3\right)_3+3NO_2+3H_2O\) (3)
\(R+4HNO_3\rightarrow M\left(NO_3\right)_3+NO+2H_2O\) (4)
Theo PTHH: \(n_{\left(-NO_3\right)\left(trong.muối\right)}=n_{NO_2}+3n_{NO}=0,9\left(mol\right)\)
=> \(m_{muối}=m_{KL}+m_{NO_3\left(trong.muối\right)}=9,9+0,9.62=65,7\left(g\right)\)
Chọn A