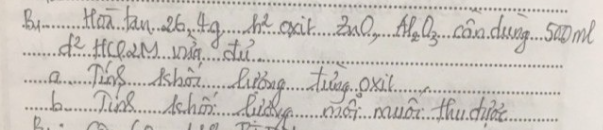a) Gọi $n_{ZnO} = a(mol) ; n_{Al_2O_3} = b(mol) \Rightarrow 81a + 102b = 26,4(1)$
$ZnO + 2HCl \to ZnCl_2 + H_2O$
$Al_2O_3 + 6HCl \to 2AlCl_3 + 3H_2O$
Theo PTHH, $n_{HCl} = 2a + 6b = 0,5.2 = 1(2)$
Từ (1)(2) suy ra a = 0,2 ; b =0,1
$m_{ZnO} = 0,2.81 = 16,2(gam)$
$m_{Al_2O_3} = 0,1.102 = 10,2(gam)$
b)
$n_{ZnCl_2} = n_{ZnO} = 0,2(mol) \Rightarrow m_{ZnCl_2} = 0,2.136 = 27,2(gam)$
$n_{AlCl_3} = 2n_{Al_2O_3} =0,2(mol) \Rightarrow m_{AlCl_3} = 0,2.133,5 = 26,7(gam)$