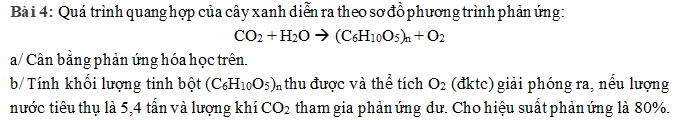a) \(6nCO_2+5nH_2O\underrightarrow{as,chất.diệp.lục}\left(C_6H_{10}O_5\right)_n+6nO_2\)
b) \(n_{H_2O\left(pư\right)}=\dfrac{5,4.10^6.80\%}{18}=240000\left(mol\right)\)
Theo PTHH: \(n_{\left(C_6H_{10}O_5\right)_n}=\dfrac{1}{5n}.n_{H_2O\left(pư\right)}=\dfrac{48000}{n}\left(mol\right)\)
=> \(m_{\left(C_6H_{10}O_5\right)_n}=\dfrac{48000}{n}.162n=7776000\left(g\right)\)
Theo PTHH: \(n_{O_2}=\dfrac{6}{5}.n_{H_2O\left(pư\right)}=288000\left(mol\right)\)
=> \(V_{O_2}=288000.22,4=6451200\left(l\right)\)