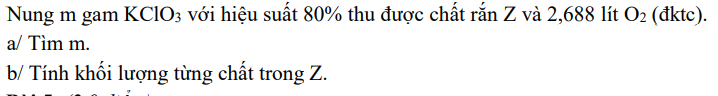a) \(n_{O_2}=\dfrac{2,688}{22,4}=0,12\left(mol\right)\)
PTHH: \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
0,08<----0,08<---0,12
\(\Rightarrow n_{KClO_3\left(tt\right)}=\dfrac{0,08.100}{80}=0,1\left(mol\right)\)
=> m = 0,1.122,5 = 12,25 (g)
b) \(Z\left\{{}\begin{matrix}KCl:0,08.74,5=5,96\left(g\right)\\KClO_3:\left(0,1-0,08\right).122,5=2,45\left(g\right)\end{matrix}\right.\)