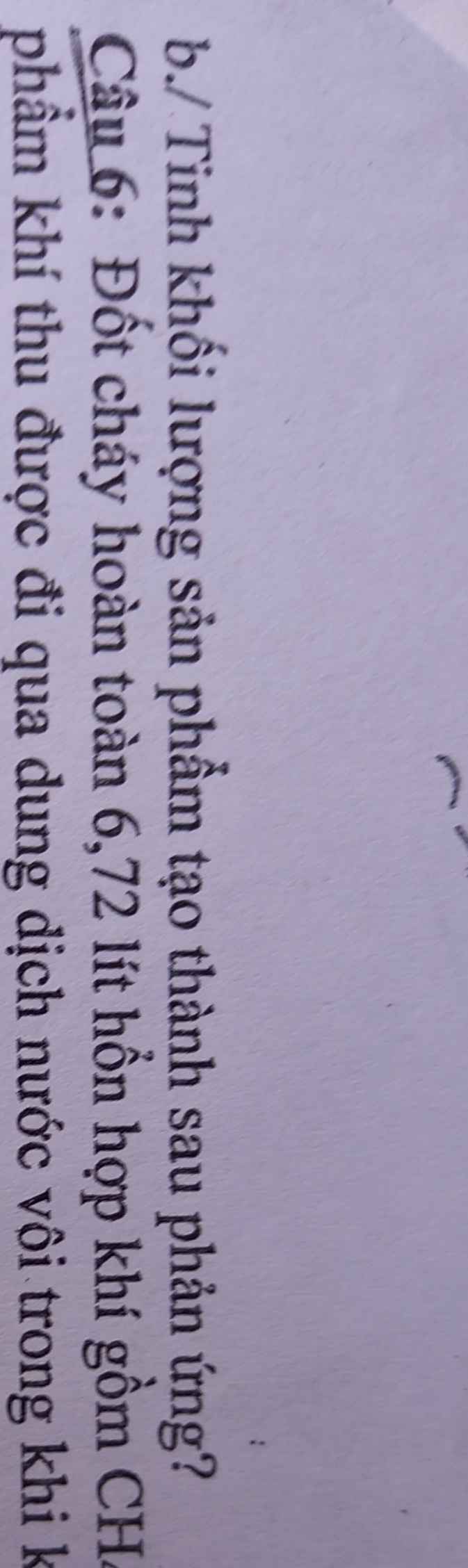\(n_{hhkhí}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(n_{Br_2}=1.0,1=0,1\left(mol\right)\)
PTHH: C2H4 + Br2 ---> C2H4Br2
0,1 0,1 0,1
\(\rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,1}{0,3}=33,33\%\\\%V_{CH_4}=100\%-33,33\%=66,67\%\end{matrix}\right.\\ \rightarrow m_{C_2H_4Br_2}=188.0,1=18,8\left(g\right)\)