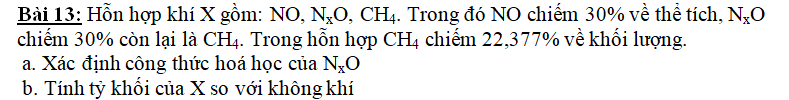
a) Giả sử có 1 mol X
=> \(\left\{{}\begin{matrix}n_{NO}=0,3\left(mol\right)\\n_{N_xO}=0,3\left(mol\right)\\n_{CH_4}=0,4\left(mol\right)\end{matrix}\right.\)
\(m_{CH_4}=0,4.16=6,4\left(g\right)\)
=> \(m_X=\dfrac{6,4.100}{22,377}=28,6\left(g\right)\)
=> \(m_{N_xO}=28,6-0,3.30-6,4=13,2\left(g\right)\)
=> \(M_{N_xO}=\dfrac{13,2}{0,3}=44\left(g/mol\right)\)
=> x = 2
=> CTHH: N2O
b) \(\overline{M}_X=\dfrac{m_X}{n_X}=\dfrac{28,6}{1}=28,6\left(g/mol\right)\)
=> \(d_{X/kk}=\dfrac{28,6}{29}=0,9862\)