1a. \(n_{O_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
- Theo PTHH ta có: \(n_{KMnO_4}=n_{O_2}\cdot\dfrac{2}{1}=0,25\cdot\dfrac{2}{1}=0,5\left(mol\right)\)
\(\Rightarrow m_{KMnO_4}=0,5.\left(39+55+16.4\right)=79\left(g\right)\)
1b. Theo PTHH ta có:
1b.i: \(n_{K_2MnO_4}=n_{O_2}\cdot\dfrac{1}{1}=0,25\cdot\dfrac{1}{1}=0,25\left(mol\right)\)
\(\Rightarrow m_{K_2MnO_4}=0,25.\left(39.2+55+16.4\right)=49,25\left(g\right)\)
1b.ii: \(n_{MnO_2}=n_{O_2}\cdot\dfrac{1}{1}=0,25\cdot\dfrac{1}{1}=0,25\left(mol\right)\)
\(\Rightarrow m_{MnO_2}=0,25.\left(55+16.2\right)=21,75\left(g\right)\)
Bài 1:
a) Số mol O2 thu được là: \(n_{O_2}\)\(=\dfrac{5,6}{22,4}=0,25\) \(mol\).
b) Phương trình hóa học: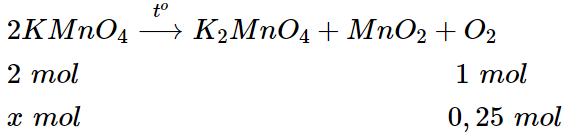
\(\dfrac{x}{2}=\dfrac{0,25}{1}\Rightarrow x=0,5\) \(mol\).
\(m_{KMnO_4}=n_{KMnO_4}.M_{KMnO_4}=0,5.158=79g\).










